当前位置:
X-MOL 学术
›
Sustain. Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The mechanism of transmethylation in anisole decomposition over Brønsted acid sites: density functional theory (DFT) study
Sustainable Energy & Fuels ( IF 5.0 ) Pub Date : 2017-08-15 00:00:00 , DOI: 10.1039/c7se00280g Jiajun Zhang 1, 2, 3, 4, 5 , Beatriz Fidalgo 5, 6, 7, 8 , Athanasios Kolios 5, 6, 7, 8 , Dekui Shen 1, 2, 3, 4 , Sai Gu 8, 9, 10, 11
Sustainable Energy & Fuels ( IF 5.0 ) Pub Date : 2017-08-15 00:00:00 , DOI: 10.1039/c7se00280g Jiajun Zhang 1, 2, 3, 4, 5 , Beatriz Fidalgo 5, 6, 7, 8 , Athanasios Kolios 5, 6, 7, 8 , Dekui Shen 1, 2, 3, 4 , Sai Gu 8, 9, 10, 11
Affiliation
|
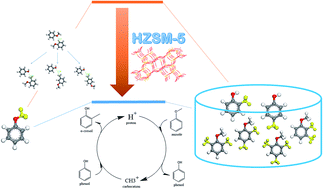
In this work, the mechanism and intrinsic reaction energy barriers of transmethylation, as the initial stage of catalytic and non-catalytic anisole decomposition, were investigated by Density Functional Theory (DFT). Molecular analyses indicated that methyl free radical transfer occurred in the absence of a catalyst, and the catalytic transmethylation over Brønsted acid sites was considered based on a dual electrophilic attack mechanism with protonation and carbocation substitution. Reaction modelling for the formation of methyl-containing compounds in both non-catalytic and catalytic anisole decomposition indicated that the energy barriers were significantly decreased in the presence of a catalyst by 60 kcal mol−1 at the most in the case of o-cresol. The results also revealed that the intrinsic transmethylation orientation preferred the ortho- and para-positions on the acceptor compounds containing oxygen-rich substituents due to their large electronegativity, and the lowest energy barrier was observed in the case of transmethylation towards the para-position of the cresol molecule (54.1 kcal mol−1).
中文翻译:

布朗斯台德酸位上苯甲醚分解中的反甲基化机理:密度泛函理论(DFT)研究
在这项工作中,通过密度泛函理论(DFT)研究了甲基转移反应的机理和内在的反应能垒,这是催化和非催化苯甲醚分解的初始阶段。分子分析表明,在没有催化剂的情况下发生了甲基自由基转移,并且基于具有质子化和碳阳离子取代的双重亲电攻击机理,考虑了布朗斯台德酸位点上的催化反甲基化。在这两个非催化和催化分解苯甲醚含甲基的化合物的形成反应建模表明能量势垒中在催化剂的存在下显著减少了60千卡摩尔-1中的情况下,在最Ô-甲酚 结果还表明,固有的甲基转移取向由于其大的电负性而优选含有富氧取代基的受体化合物上的邻位和对位,并且在向甲基对位的对位转移时观察到最低的能垒。甲酚分子(54.1 kcal mol -1)。
更新日期:2017-08-29
中文翻译:

布朗斯台德酸位上苯甲醚分解中的反甲基化机理:密度泛函理论(DFT)研究
在这项工作中,通过密度泛函理论(DFT)研究了甲基转移反应的机理和内在的反应能垒,这是催化和非催化苯甲醚分解的初始阶段。分子分析表明,在没有催化剂的情况下发生了甲基自由基转移,并且基于具有质子化和碳阳离子取代的双重亲电攻击机理,考虑了布朗斯台德酸位点上的催化反甲基化。在这两个非催化和催化分解苯甲醚含甲基的化合物的形成反应建模表明能量势垒中在催化剂的存在下显著减少了60千卡摩尔-1中的情况下,在最Ô-甲酚 结果还表明,固有的甲基转移取向由于其大的电负性而优选含有富氧取代基的受体化合物上的邻位和对位,并且在向甲基对位的对位转移时观察到最低的能垒。甲酚分子(54.1 kcal mol -1)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号