当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Aberrantly Large Single-Channel Conductance of Polyhistidine Arm-Containing Protein Nanopores
Biochemistry ( IF 2.9 ) Pub Date : 2017-08-28 00:00:00 , DOI: 10.1021/acs.biochem.7b00577 Avinash Kumar Thakur 1, 2 , Motahareh Ghahari Larimi 1 , Kristin Gooden 3 , Liviu Movileanu 1, 2, 4
Biochemistry ( IF 2.9 ) Pub Date : 2017-08-28 00:00:00 , DOI: 10.1021/acs.biochem.7b00577 Avinash Kumar Thakur 1, 2 , Motahareh Ghahari Larimi 1 , Kristin Gooden 3 , Liviu Movileanu 1, 2, 4
Affiliation
|
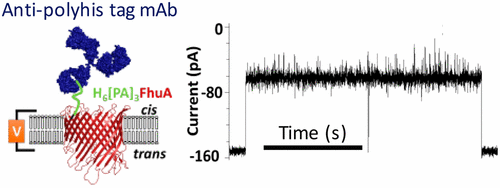
There have been only a few studies reporting on the impact of polyhistidine affinity tags on the structure, function, and dynamics of proteins. Because of the relatively short size of the tags, they are often thought to have little or no effect on the conformation or activity of a protein. Here, using membrane protein design and single-molecule electrophysiology, we determined that the presence of a hexahistidine arm at the N-terminus of a truncated FhuA-based protein nanopore, leaving the C-terminus untagged, produces an unusual increase in the unitary conductance to ∼8 nS in 1 M KCl. To the best of our knowledge, this is the largest single-channel conductance ever recorded with a monomeric β-barrel outer membrane protein. The hexahistidine arm was captured by an anti-polyhistidine tag monoclonal antibody added to the side of the channel-forming protein addition, but not to the opposite side, documenting that this truncated FhuA-based protein nanopore inserts into a planar lipid bilayer with a preferred orientation. This finding is in agreement with the protein insertion in vivo, in which the large loops face the extracellular side of the membrane. The aberrantly large single-channel conductance, likely induced by a greater cross-sectional area of the pore lumen, along with the vectorial insertion into a lipid membrane, will have profound implications for further developments of engineered protein nanopores.
中文翻译:

多组氨酸臂蛋白纳米孔的异常大单通道电导。
仅有少数研究报道了多组氨酸亲和标签对蛋白质的结构,功能和动力学的影响。由于标签的尺寸相对较短,通常认为它们对蛋白质的构象或活性几乎没有影响。在这里,使用膜蛋白设计和单分子电生理学,我们确定在被截断的基于FhuA的蛋白纳米孔的N末端存在一个六组氨酸臂,而C末端未加标记,这会导致单位电导率异常增加。在1 M KCl中至约8 nS。据我们所知,这是有记录的单体β桶外膜蛋白最大的单通道电导。六组氨酸臂被抗多组氨酸标签单克隆抗体捕获,该抗体被添加到通道形成蛋白添加的一侧,但没有添加到另一侧,证明该截短的基于FhuA的蛋白纳米孔插入到平面脂质双层中,具有较好的方向。这个发现与蛋白质插入是一致的在体内,其中大环面向膜的细胞外侧。异常大的单通道电导,可能是由更大的孔腔横截面面积诱导的,以及在脂质膜中的矢量插入,将对工程蛋白纳米孔的进一步发展产生深远的影响。
更新日期:2017-08-29
中文翻译:

多组氨酸臂蛋白纳米孔的异常大单通道电导。
仅有少数研究报道了多组氨酸亲和标签对蛋白质的结构,功能和动力学的影响。由于标签的尺寸相对较短,通常认为它们对蛋白质的构象或活性几乎没有影响。在这里,使用膜蛋白设计和单分子电生理学,我们确定在被截断的基于FhuA的蛋白纳米孔的N末端存在一个六组氨酸臂,而C末端未加标记,这会导致单位电导率异常增加。在1 M KCl中至约8 nS。据我们所知,这是有记录的单体β桶外膜蛋白最大的单通道电导。六组氨酸臂被抗多组氨酸标签单克隆抗体捕获,该抗体被添加到通道形成蛋白添加的一侧,但没有添加到另一侧,证明该截短的基于FhuA的蛋白纳米孔插入到平面脂质双层中,具有较好的方向。这个发现与蛋白质插入是一致的在体内,其中大环面向膜的细胞外侧。异常大的单通道电导,可能是由更大的孔腔横截面面积诱导的,以及在脂质膜中的矢量插入,将对工程蛋白纳米孔的进一步发展产生深远的影响。










































 京公网安备 11010802027423号
京公网安备 11010802027423号