当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Experimental and Theoretical Mechanistic Investigation on the Catalytic CO2 Hydrogenation to Formate by a Carboxylate-Functionalized Bis(N-heterocyclic carbene) Zwitterionic Iridium(I) Compound
Organometallics ( IF 2.5 ) Pub Date : 2017-08-28 00:00:00 , DOI: 10.1021/acs.organomet.7b00509 Raquel Puerta-Oteo 1, 2 , Markus Hölscher 2 , M. Victoria Jiménez 1 , Walter Leitner 2 , Vincenzo Passarelli 1, 3 , Jesús J. Pérez-Torrente 1
Organometallics ( IF 2.5 ) Pub Date : 2017-08-28 00:00:00 , DOI: 10.1021/acs.organomet.7b00509 Raquel Puerta-Oteo 1, 2 , Markus Hölscher 2 , M. Victoria Jiménez 1 , Walter Leitner 2 , Vincenzo Passarelli 1, 3 , Jesús J. Pérez-Torrente 1
Affiliation
|
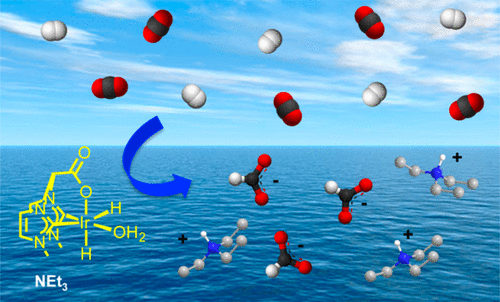
The bis-imidazolium salt, 1,1-bis(N-methylimidazolium) acetate bromide, is a convenient precursor for the synthesis of zwitterionic iridium(I) [Ir(cod){(MeIm)2CHCOO}] and cationic iridium(III) [IrH(cod){(MeIm)2CHCOO}]+ compounds (MeIm = 3-methylimidazol-2-yliden-1-yl) having a carboxylate-functionalized bis(NHC) ligand. The [Ir(cod){(MeIm)2CHCOO}] compound catalyzes the hydrogenation of CO2 to formate in water using NEt3 as base, reaching turnover numbers of approximately 1500. Reactivity studies have shown that activation of the catalyst precursor involves the reaction with H2 in a multistep process that under catalytic conditions results in the formation of a dihydrido iridium(III) octahedral [IrH2(H2O){(MeIm)2CHCOO}] species stabilized by the κ3-C,C′,O coordination of the ligand. DFT studies on the mechanism were carried out to elucidate two possible roles of the base. In the first one, NEt3 neutralizes only the produced formic acid, whereas in the second it assists the proton transfer in heterolytic cleavage of the H2 molecule. Although this base-involved mechanism is more favorable in that it exhibits a lower energy span for the overall reaction, the energy barrier obtained from kinetic experiments suggests that both mechanisms could be operative under the experimental reaction conditions.
中文翻译:

羧酸盐官能化双(N-杂环卡宾)两性离子铱(I)化合物催化CO 2加氢生成甲酸酯的实验和理论机理研究
双咪唑鎓盐,即1,1-双(N-甲基咪唑鎓)乙酸溴化物,是合成两性离子铱(I)[Ir(cod){(MeIm)2 CHCOO}]和阳离子铱(III )的便捷前体)[IrH(cod){(MeIm)2 CHCOO}] +具有羧酸盐官能化的双(NHC)配体的化合物(MeIm = 3-甲基咪唑-2-基亚基-1-基)。[Ir(cod){(MeIm)2 CHCOO}]化合物使用NEt 3作为碱催化水中的CO 2加氢生成甲酸,达到约1500的周转率。反应性研究表明,催化剂前体的活化涉及与H 2反应在一个多步骤过程,在催化条件下导致形成一个dihydrido铱(III)的八面体[IRH 2(H 2 O){(MEIM)2 CHCOO}]物种由κ稳定3 - ç,C' ,Ò协调配体。进行了DFT机制研究,以阐明该碱基的两个可能作用。在第一个反应中,NEt 3仅中和产生的甲酸,而在第二个反应中,它协助质子转移H 2的异质裂解。分子。尽管这种涉及碱的机理在整个反应过程中表现出较低的能量跨度是更有利的,但是从动力学实验获得的能垒表明这两种机理都可以在实验反应条件下起作用。
更新日期:2018-02-02
中文翻译:

羧酸盐官能化双(N-杂环卡宾)两性离子铱(I)化合物催化CO 2加氢生成甲酸酯的实验和理论机理研究
双咪唑鎓盐,即1,1-双(N-甲基咪唑鎓)乙酸溴化物,是合成两性离子铱(I)[Ir(cod){(MeIm)2 CHCOO}]和阳离子铱(III )的便捷前体)[IrH(cod){(MeIm)2 CHCOO}] +具有羧酸盐官能化的双(NHC)配体的化合物(MeIm = 3-甲基咪唑-2-基亚基-1-基)。[Ir(cod){(MeIm)2 CHCOO}]化合物使用NEt 3作为碱催化水中的CO 2加氢生成甲酸,达到约1500的周转率。反应性研究表明,催化剂前体的活化涉及与H 2反应在一个多步骤过程,在催化条件下导致形成一个dihydrido铱(III)的八面体[IRH 2(H 2 O){(MEIM)2 CHCOO}]物种由κ稳定3 - ç,C' ,Ò协调配体。进行了DFT机制研究,以阐明该碱基的两个可能作用。在第一个反应中,NEt 3仅中和产生的甲酸,而在第二个反应中,它协助质子转移H 2的异质裂解。分子。尽管这种涉及碱的机理在整个反应过程中表现出较低的能量跨度是更有利的,但是从动力学实验获得的能垒表明这两种机理都可以在实验反应条件下起作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号