当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pharmacological Evaluation of Novel Bioisosteres of an Adamantanyl Benzamide P2X7 Receptor Antagonist
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2017-08-25 00:00:00 , DOI: 10.1021/acschemneuro.7b00272 Shane M. Wilkinson , Melissa L. Barron , James O’Brien-Brown , Bieneke Janssen 1 , Leanne Stokes 2 , Eryn L. Werry , Mansoor Chishty 3 , Kristen K. Skarratt , Jennifer A. Ong , David E. Hibbs , Danielle J. Vugts 1 , Stephen Fuller , Albert D. Windhorst 1 , Michael Kassiou
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2017-08-25 00:00:00 , DOI: 10.1021/acschemneuro.7b00272 Shane M. Wilkinson , Melissa L. Barron , James O’Brien-Brown , Bieneke Janssen 1 , Leanne Stokes 2 , Eryn L. Werry , Mansoor Chishty 3 , Kristen K. Skarratt , Jennifer A. Ong , David E. Hibbs , Danielle J. Vugts 1 , Stephen Fuller , Albert D. Windhorst 1 , Michael Kassiou
Affiliation
|
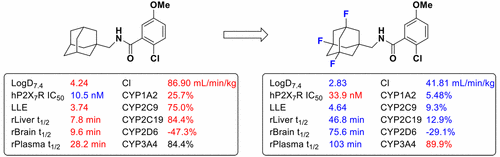
Adamantanyl benzamide 1 was identified as a potent P2X7R antagonist but failed to progress further due to poor metabolic stability. We describe the synthesis and SAR of a series of bioisosteres of benzamide 1 to explore improvements in the pharmacological properties of this lead. Initial efforts investigated a series of heteroaromatic bioisosteres, which demonstrated improved physicochemical properties but reduced P2X7R antagonism. Installation of bioisosteric fluorine on the adamantane bridgeheads was well tolerated and led to a series of bioisosteres with improved physicochemical properties and metabolic stability. Trifluorinated benzamide 34 demonstrated optimal physicochemical parameters, superior metabolic stability (ten times longer than lead benzamide 1), and an improved physicokinetic profile and proved effective in the presence of several known P2X7R polymorphisms.
中文翻译:

精金刚烷苯甲酰胺P2X 7受体拮抗剂的新型生物甾体的药理评价
精金刚烷苯甲酰胺1被确定为有效的P2X 7 R拮抗剂,但由于代谢稳定性差而未能进一步发展。我们描述了一系列苯甲酰胺1的生物等排体的合成和SAR,以探索这种铅的药理特性的改善。最初的努力研究了一系列杂芳族生物等排体,它们显示出改善的理化性质,但减少了P2X 7 R拮抗作用。在金刚烷桥头上安装了生物立体异构的氟是很好的耐受性,并导致了一系列具有改善的理化性质和代谢稳定性的生物立体异构体。三氟苯甲酰胺34证明了最佳的物理化学参数,优异的代谢稳定性(比苯甲酰胺铅1的长十倍)和改善的物理动力学特性,并被证明在存在几种已知的P2X 7 R多态性时有效。
更新日期:2017-08-25
中文翻译:

精金刚烷苯甲酰胺P2X 7受体拮抗剂的新型生物甾体的药理评价
精金刚烷苯甲酰胺1被确定为有效的P2X 7 R拮抗剂,但由于代谢稳定性差而未能进一步发展。我们描述了一系列苯甲酰胺1的生物等排体的合成和SAR,以探索这种铅的药理特性的改善。最初的努力研究了一系列杂芳族生物等排体,它们显示出改善的理化性质,但减少了P2X 7 R拮抗作用。在金刚烷桥头上安装了生物立体异构的氟是很好的耐受性,并导致了一系列具有改善的理化性质和代谢稳定性的生物立体异构体。三氟苯甲酰胺34证明了最佳的物理化学参数,优异的代谢稳定性(比苯甲酰胺铅1的长十倍)和改善的物理动力学特性,并被证明在存在几种已知的P2X 7 R多态性时有效。











































 京公网安备 11010802027423号
京公网安备 11010802027423号