Journal of Controlled Release ( IF 10.5 ) Pub Date : 2017-08-24 , DOI: 10.1016/j.jconrel.2017.08.030 Mona Alibolandi , Khalil Abnous , Marzieh Mohammadi , Farzin Hadizadeh , Fatemeh Sadeghi , Sahar Taghavi , Mahmoud Reza Jaafari , Mohammad Ramezani
|
Due to the severe cardiotoxicity of doxorubicin, its usage is limited. This shortcoming could be overcome by modifying pharmacokinetics of the drugs via preparation of various nanoplatforms.
Doxil, a well-known FDA-approved nanoplatform of doxorubicin as antineoplastic agent, is frequently used in clinics in order to reduce cardiotoxicity of doxorubicin. Since Doxil shows some shortcomings in clinics including hand and food syndrome and very slow release pattern thus, there is a demand for the development and preparation of new doxorubicin nanoformulation with fewer side effects.
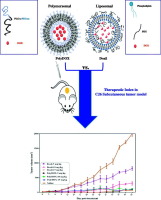
The new formulation of the doxorubicin, synthesized previously by our group was extensively examined in the current study. This new formulation is doxorubicin encapsulated in PEG-PLGA polymersomes (PolyDOX). The main aim of the study was to compare the distribution and treatment efficacy of a new doxorubicin-polymersomal formulation (PolyDOX) with regular liposomal formulation (Doxil-mimic) in murine colon adenocarcinoma model. Additionally, the pathological, hematological changes, pharmacodynamics, biodistribution, tolerated dose and survival rate in vivo were evaluated and compared.
Murine colon cancer model was induced by subcutaneous inoculation of BALB/c mice with C26 cells. Afterwards, either Doxil-mimic or PolyDOX was administered intravenously.
The obtained results from biodistribution study showed a remarkable difference in the distribution of drugs in murine organs. In this regard, Doxil-mimic exhibited prolonged (48 h) presence within liver tissues while PolyDOX preferentially accumulate in tumor and the presence in liver 48 h post-treatment was significantly lower than that of Doxil-mimic.
Obtained results demonstrated comparable final length of life for mice receiving either Doxil-mimic or PolyDOX formulations whereas tolerated dose of mice receiving Doxil-mimic was remarkably higher than those receiving PolyDOX.
Therapeutic efficacy of formulation in term of tumor growth rate after one injection of formulations (5 mg/kg, 10 mg/kg or 15 mg/kg) demonstrated better efficacy at lower dose for PolyDOX.
Analysis of Kaplan Meier curve was in favor of both formulations in their treatment–dose. Pathological and hematological surveys of mice treated with both formulations did not show considerable difference except for a small atrophy in liver observed after successive administration of Doxil-mimic.
It could be concluded that PolyDOX can potentially limit off-site effects of Doxil due to its biodegradability and sustained release properties while it exhibited favorable safety profile comparable to Doxil.
中文翻译:

阿霉素多体制剂与阿霉素模拟制剂的广泛临床前研究
由于阿霉素的严重心脏毒性,其使用受到限制。通过制备各种纳米平台来改变药物的药代动力学可以克服这一缺点。
为了降低阿霉素的心脏毒性,Doxil是一种著名的FDA批准的阿霉素作为抗肿瘤药的纳米平台,在临床上经常使用。由于Doxil在临床上显示出一些缺点,包括手和食物综合症以及释放速度非常缓慢,因此,需要开发和制备副作用少的新型阿霉素纳米制剂。
我们小组先前合成的阿霉素新制剂在本研究中已得到广泛研究。这种新配方是将阿霉素封装在PEG-PLGA聚合物囊泡(PolyDOX)中。该研究的主要目的是在鼠结肠腺癌模型中比较一种新的阿霉素-多聚体制剂(PolyDOX)和常规脂质体制剂(Doxil-mimic)的分布和治疗效果。另外,评估并比较了体内的病理,血液学变化,药效学,生物分布,耐受剂量和存活率。
通过用C26细胞皮下接种BALB / c小鼠来诱导鼠结肠癌模型。之后,静脉内施用Doxil-mimic或PolyDOX。
生物分布研究获得的结果表明,药物在鼠器官中的分布存在显着差异。在这方面,Doxil-mimic表现出在肝脏组织中延长的时间(48 h),而PolyDOX优先在肿瘤中蓄积,并且治疗后48 h在肝脏中的存在显着低于Doxil-micmic。
获得的结果表明,接受Doxil模拟或PolyDOX制剂的小鼠的最终寿命相当,而接受Doxil模拟的小鼠的耐受剂量明显高于接受PolyDOX的小鼠。
在一剂制剂(5 mg / kg,10 mg / kg或15 mg / kg)注射后,就肿瘤生长率而言,该制剂的治疗功效证明了在较低剂量下对PolyDOX的更好的功效。
Kaplan Meier曲线的分析有利于两种制剂的治疗剂量。用两种制剂治疗的小鼠的病理学和血液学调查结果均未发现明显差异,只是在连续服用Doxil-mic药物后观察到肝脏出现了微小的萎缩。
可以得出结论,由于PolyDOX的生物降解性和持续释放特性,它可以潜在地限制Doxil的异位作用,而与Doxil相比,它具有良好的安全性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号