当前位置:
X-MOL 学术
›
Water Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly selective transformation of ammonia nitrogen to N2 based on a novel solar-driven photoelectrocatalytic-chlorine radical reactions system
Water Research ( IF 11.4 ) Pub Date : 2017-08-24 , DOI: 10.1016/j.watres.2017.08.053 Youzhi Ji , Jing Bai , Jinhua Li , Tao Luo , Li Qiao , Qingyi Zeng , Baoxue Zhou
Water Research ( IF 11.4 ) Pub Date : 2017-08-24 , DOI: 10.1016/j.watres.2017.08.053 Youzhi Ji , Jing Bai , Jinhua Li , Tao Luo , Li Qiao , Qingyi Zeng , Baoxue Zhou
|
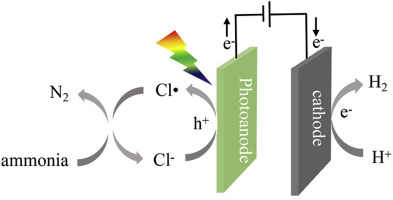
A highly selective method for transforming ammonia nitrogen to N2 was proposed, based on a novel solar-driven photoelectrocatalytic-chlorine radical reactions (PEC-chlorine) system. The PEC-chlorine system was facilitated by a visible light response WO3 nanoplate array (NPA) electrode in an ammonia solution containing chloride ions (Cl−). Under illumination, photoholes from WO3 promote the oxidation of Cl− to chlorine radical (Cl). This radical can selectively transform ammonia nitrogen to N2 (79.9%) and NO3− (19.2%), similar to the breakpoint chlorination reaction. The ammonia nitrogen removal efficiency increased from 10.6% (PEC without Cl−) to 99.9% with the PEC-chlorine system within 90 min operation, which can be attributed to the cyclic reactions between Cl−/Cl and the reaction intermediates (NH2
and the reaction intermediates (NH2 ,
,  NHCl, etc.) that expand the degradation reactions from the surface of the electrodes to the whole solution system. Moreover, Cl
NHCl, etc.) that expand the degradation reactions from the surface of the electrodes to the whole solution system. Moreover, Cl is the main radical species contributing to the transformation of ammonia nitrogen to N2, which is confirmed by the tBuOH capture experiment. Compared to conventional breakpoint chlorination, the PEC-chlorine system is a more economical and efficient means for ammonia nitrogen degradation because of the fast removal rate, no additional chlorine cost, and its use of clean energy (since it is solar-driven).
is the main radical species contributing to the transformation of ammonia nitrogen to N2, which is confirmed by the tBuOH capture experiment. Compared to conventional breakpoint chlorination, the PEC-chlorine system is a more economical and efficient means for ammonia nitrogen degradation because of the fast removal rate, no additional chlorine cost, and its use of clean energy (since it is solar-driven).
中文翻译:

基于新型太阳能驱动的光电催化氯自由基反应体系的高选择性氨氮转化为N 2
提出了一种新型的太阳能驱动的光电催化氯自由基反应体系,将氨氮转化为N 2的高选择性方法。在PEC -氯系统是由一可见光响应WO促进3在含有氯离子(CL氨溶液纳米板阵列(NPA)电极- )。在照明下,从WO光穴3促进Cl组成的氧化-对氯基(氯)。该基团可以有选择地变换氨氮到N 2(79.9%)和NO 3 -(19.2%),类似于断点氯化反应。氨氮去除率由10.6%(PEC无氯增加- )至99.9%,90分钟内操作的PEC-氯系统,其可以归因于氯之间的循环反应- /氯 和反应中间体(NH 2
和反应中间体(NH 2 ,
,  NHCl等),将降解反应从电极表面扩展到整个溶液系统。而且,Cl
NHCl等),将降解反应从电极表面扩展到整个溶液系统。而且,Cl tBuOH捕获实验证实,它是导致氨氮转化为N 2的主要自由基。与传统的断点氯化法相比,PEC氯化物系统是一种更经济,更有效的氨氮降解方法,因为它的去除速度快,无额外的氯成本,并且使用清洁能源(因为它是太阳能驱动的)。
tBuOH捕获实验证实,它是导致氨氮转化为N 2的主要自由基。与传统的断点氯化法相比,PEC氯化物系统是一种更经济,更有效的氨氮降解方法,因为它的去除速度快,无额外的氯成本,并且使用清洁能源(因为它是太阳能驱动的)。
更新日期:2017-08-25
 and the reaction intermediates (NH2
and the reaction intermediates (NH2 ,
,  NHCl, etc.) that expand the degradation reactions from the surface of the electrodes to the whole solution system. Moreover, Cl
NHCl, etc.) that expand the degradation reactions from the surface of the electrodes to the whole solution system. Moreover, Cl is the main radical species contributing to the transformation of ammonia nitrogen to N2, which is confirmed by the tBuOH capture experiment. Compared to conventional breakpoint chlorination, the PEC-chlorine system is a more economical and efficient means for ammonia nitrogen degradation because of the fast removal rate, no additional chlorine cost, and its use of clean energy (since it is solar-driven).
is the main radical species contributing to the transformation of ammonia nitrogen to N2, which is confirmed by the tBuOH capture experiment. Compared to conventional breakpoint chlorination, the PEC-chlorine system is a more economical and efficient means for ammonia nitrogen degradation because of the fast removal rate, no additional chlorine cost, and its use of clean energy (since it is solar-driven).
中文翻译:

基于新型太阳能驱动的光电催化氯自由基反应体系的高选择性氨氮转化为N 2
提出了一种新型的太阳能驱动的光电催化氯自由基反应体系,将氨氮转化为N 2的高选择性方法。在PEC -氯系统是由一可见光响应WO促进3在含有氯离子(CL氨溶液纳米板阵列(NPA)电极- )。在照明下,从WO光穴3促进Cl组成的氧化-对氯基(氯)。该基团可以有选择地变换氨氮到N 2(79.9%)和NO 3 -(19.2%),类似于断点氯化反应。氨氮去除率由10.6%(PEC无氯增加- )至99.9%,90分钟内操作的PEC-氯系统,其可以归因于氯之间的循环反应- /氯
 和反应中间体(NH 2
和反应中间体(NH 2 ,
,  NHCl等),将降解反应从电极表面扩展到整个溶液系统。而且,Cl
NHCl等),将降解反应从电极表面扩展到整个溶液系统。而且,Cl tBuOH捕获实验证实,它是导致氨氮转化为N 2的主要自由基。与传统的断点氯化法相比,PEC氯化物系统是一种更经济,更有效的氨氮降解方法,因为它的去除速度快,无额外的氯成本,并且使用清洁能源(因为它是太阳能驱动的)。
tBuOH捕获实验证实,它是导致氨氮转化为N 2的主要自由基。与传统的断点氯化法相比,PEC氯化物系统是一种更经济,更有效的氨氮降解方法,因为它的去除速度快,无额外的氯成本,并且使用清洁能源(因为它是太阳能驱动的)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号