当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cathepsin-Mediated Cleavage of Peptides from Peptide Amphiphiles Leads to Enhanced Intracellular Peptide Accumulation
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2017-08-24 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00364 Handan Acar 1, 2 , Ravand Samaeekia 1, 2 , Mathew R. Schnorenberg 1, 2, 3 , Dibyendu K. Sasmal 1 , Jun Huang 1 , Matthew V. Tirrell 1, 4 , James L. LaBelle 2
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2017-08-24 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00364 Handan Acar 1, 2 , Ravand Samaeekia 1, 2 , Mathew R. Schnorenberg 1, 2, 3 , Dibyendu K. Sasmal 1 , Jun Huang 1 , Matthew V. Tirrell 1, 4 , James L. LaBelle 2
Affiliation
|
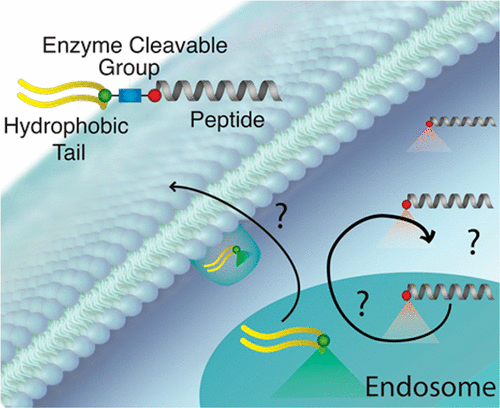
Peptides synthesized in the likeness of their native interaction domain(s) are natural choices to target protein–protein interactions (PPIs) due to their fidelity of orthostatic contact points between binding partners. Despite therapeutic promise, intracellular delivery of biofunctional peptides at concentrations necessary for efficacy remains a formidable challenge. Peptide amphiphiles (PAs) provide a facile method of intracellular delivery and stabilization of bioactive peptides. PAs consisting of biofunctional peptide headgroups linked to hydrophobic alkyl lipid-like tails prevent peptide hydrolysis and proteolysis in circulation, and PA monomers are internalized via endocytosis. However, endocytotic sequestration and steric hindrance from the lipid tail are two major mechanisms that limit PA efficacy to target intracellular PPIs. To address these problems, we have constructed a PA platform consisting of cathepsin-B cleavable PAs in which a selective p53-based inhibitory peptide is cleaved from its lipid tail within endosomes, allowing for intracellular peptide accumulation and extracellular recycling of the lipid moiety. We monitor for cleavage and follow individual PA components in real time using a Förster resonance energy transfer (FRET)-based tracking system. Using this platform, we provide a better understanding and quantification of cellular internalization, trafficking, and endosomal cleavage of PAs and of the ultimate fates of each component.
中文翻译:

组织蛋白酶介导的肽两亲物对肽的切割导致增强的细胞内肽积累。
以其天然相互作用域相似的方式合成的肽是天然的选择,因为它们具有结合伙伴之间的直立接触点的保真性,因此是靶向蛋白质-蛋白质相互作用(PPI)的自然选择。尽管有治疗前景,但以功效所必需的浓度在细胞内递送生物功能肽仍然是一个艰巨的挑战。肽两亲物(PAs)提供了一种简便的细胞内递送和生物活性肽稳定化的方法。PAs由与疏水性烷基脂质样尾巴相连的生物功能肽头基组成,可防止肽在循环中水解和蛋白水解,并且PA单体通过内吞作用而被内在化。但是,内吞性螯合和脂质尾部的位阻是限制PA疗效靶向细胞内PPI的两个主要机制。为了解决这些问题,我们构建了一个由组织蛋白酶B可裂解的PA组成的PA平台,其中选择性的基于p53的抑制性肽从脂质体的脂质尾部裂解,从而允许细胞内肽积累和脂质部分的细胞外循环。我们使用基于Förster共振能量转移(FRET)的跟踪系统来监测裂解并实时跟踪各个PA成分。使用该平台,我们可以更好地理解和量化PA的细胞内在化,运输和内体切割,以及每个成分的最终命运。允许细胞内肽积累和脂质部分的细胞外再循环。我们使用基于Förster共振能量转移(FRET)的跟踪系统来监测裂解并实时跟踪各个PA成分。使用该平台,我们可以更好地理解和量化PA的细胞内在化,运输和内体切割,以及每个成分的最终命运。允许细胞内肽积累和脂质部分的细胞外再循环。我们使用基于Förster共振能量转移(FRET)的跟踪系统来监测裂解并实时跟踪各个PA成分。使用该平台,我们可以更好地理解和量化PA的细胞内在化,运输和内体切割,以及每个成分的最终命运。
更新日期:2017-08-25
中文翻译:

组织蛋白酶介导的肽两亲物对肽的切割导致增强的细胞内肽积累。
以其天然相互作用域相似的方式合成的肽是天然的选择,因为它们具有结合伙伴之间的直立接触点的保真性,因此是靶向蛋白质-蛋白质相互作用(PPI)的自然选择。尽管有治疗前景,但以功效所必需的浓度在细胞内递送生物功能肽仍然是一个艰巨的挑战。肽两亲物(PAs)提供了一种简便的细胞内递送和生物活性肽稳定化的方法。PAs由与疏水性烷基脂质样尾巴相连的生物功能肽头基组成,可防止肽在循环中水解和蛋白水解,并且PA单体通过内吞作用而被内在化。但是,内吞性螯合和脂质尾部的位阻是限制PA疗效靶向细胞内PPI的两个主要机制。为了解决这些问题,我们构建了一个由组织蛋白酶B可裂解的PA组成的PA平台,其中选择性的基于p53的抑制性肽从脂质体的脂质尾部裂解,从而允许细胞内肽积累和脂质部分的细胞外循环。我们使用基于Förster共振能量转移(FRET)的跟踪系统来监测裂解并实时跟踪各个PA成分。使用该平台,我们可以更好地理解和量化PA的细胞内在化,运输和内体切割,以及每个成分的最终命运。允许细胞内肽积累和脂质部分的细胞外再循环。我们使用基于Förster共振能量转移(FRET)的跟踪系统来监测裂解并实时跟踪各个PA成分。使用该平台,我们可以更好地理解和量化PA的细胞内在化,运输和内体切割,以及每个成分的最终命运。允许细胞内肽积累和脂质部分的细胞外再循环。我们使用基于Förster共振能量转移(FRET)的跟踪系统来监测裂解并实时跟踪各个PA成分。使用该平台,我们可以更好地理解和量化PA的细胞内在化,运输和内体切割,以及每个成分的最终命运。











































 京公网安备 11010802027423号
京公网安备 11010802027423号