当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, Structures, and Properties of Luminescent (C∧N∧C)gold(III) Alkyl Complexes: Correlation between Photoemission Energies and C–H Acidity
Organometallics ( IF 2.5 ) Pub Date : 2017-08-24 00:00:00 , DOI: 10.1021/acs.organomet.7b00439 Julio Fernandez-Cestau 1 , Benoı̂t Bertrand 1 , Anna Pintus 1 , Manfred Bochmann 1
Organometallics ( IF 2.5 ) Pub Date : 2017-08-24 00:00:00 , DOI: 10.1021/acs.organomet.7b00439 Julio Fernandez-Cestau 1 , Benoı̂t Bertrand 1 , Anna Pintus 1 , Manfred Bochmann 1
Affiliation
|
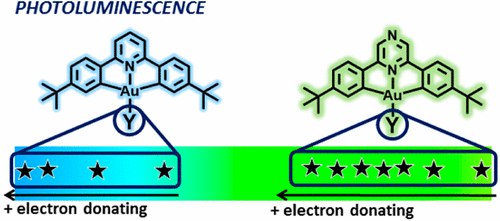
The reaction of (C∧Npz∧C)AuCl with C–H acidic compounds H2CR1R2 in the presence of base readily affords the corresponding alkyl complexes (C∧Npz∧C)AuCHR1R2 (2a, R1 = R2 = CN; 3a, R1 = R2 = C(O)Me; 4, R1 = R2 = CO2Et; 5, R1 = R2 = C(O)Ph; 6a, R1 = H, R2 = C(O)Me). The analogous pyridine-pincer complexes 2b, 3b, and 6b were obtained similarly (C∧Npz∧C = 2,6-bis(4′-tBuC6H3)2pyrazine dianion; C∧Npy∧C = 2,6-bis(4′-tBuC6H3)2pyridine dianion). The reactions of (C∧Npz∧C)AuOAcF (7a) and (C∧Npy∧C)AuOAcF (7b) with MeMgI gave the methyl complexes (C∧Npz∧C)AuMe (8a) and (C∧Npy∧C)AuMe (8b), respectively. The crystal structures of 2a,b, 3a, 6a, and 7a have been determined. The photophysical properties of the new complexes and those of the previously reported gold hydride (C∧Npz∧C)AuH (AuH) are reported. The lower-energy absorption and the emission maxima follow the energy sequence 2a < 3a < 4 < 5 < 6a < AuH ≈ 8a for the pyrazine series and 2b < 3b < 6b ≈ 8b for the pyridine series. These values provide a correlation with the pKa values of the corresponding ancillary ligand precursors. In agreement with DFT calculations, the emissions are assigned to 3IL(C∧N∧C)/3ILCT ((C6H4tBu-4′) → pz/py*) transitions, dominated by the HOMO and the LUMO orbitals. The LUMO is located in the heterocycle (py/pz) in a position trans to the ancillary ligand, which makes this orbital more sensitive to the electronic nature of the ancillary ligand than the HOMO. The calculations establish that the charge transfer from the tBuC6H3 ligand fragments to the central heterocycle ring in the dominant transition explains the modulation of the properties with the σ-donor characteristics of the alkyl or hydride ligands.
中文翻译:

的合成,结构,和发光的属性(C ∧ Ñ ∧ C)金(III)配合物的烷基:光电发射能和C-H酸度之间的相关性
的反应(C ∧ Ñ PZ ∧ C)AUCL与C-H酸性化合物ħ 2 CR 1 - [R 2在碱的存在下容易地得到相应的烷基络合物(C ∧ Ñ PZ ∧ C)AuCHR 1 - [R 2(2A, R 1= R 2= CN;3a,R 1= R 2= C(O)Me;4,R 1= R 2= CO 2 Et;5,R 1= R 2= C(O)Ph;R 1= R 2= CO 2 Et 。在图6a中,R 1= H,R 2= C(O)Me)。类似吡啶钳形络合物2B,3B,和图6B同样地得到(C ∧ Ñ PZ ∧ C = 2,6-双(4'-吨BUC 6 ħ 3)2吡嗪的二价阴离子;Ç ∧ Ñ PY ∧ C = 2 ,6-双(4' - t BuC 6 H 3)2吡啶二阴离子)。的(C反应∧ Ñ PZ ∧ C)AuOAc ˚F(7A)和(C∧ Ñ PY ∧ C)AuOAc ˚F(图7b)与MeMgI(C得到甲基络合物∧ Ñ PZ ∧ C)AuMe(8A()和C ∧ Ñ PY ∧ C)AuMe(8B分别地)。已经确定了2a,b,3a,6a和7a的晶体结构。新络合物的光物理特性以及那些以前报道的金的氢化物(C ∧ Ñ PZ ∧ C)AUH(AUH)的报告。较低的能量吸收和发射最大值按照能量序列2A < 3A < 4 < 5 < 6A < AUH ≈ 8A为吡嗪系列和图2b < 3B < 6B ≈ 8B为吡啶系列。这些值提供了与相应的辅助配体前体的p K a值的相关性。在DFT计算协议,排放被分配给3 IL(C ∧ Ñ ∧ C)/ 3 ILCT((C 6 H ^由HOMO和LUMO轨道控制的4 t Bu-4')→pz / py *)跃迁。LUMO位于杂环(py / pz)中,其位置与辅助配体相反,与HOMO相比,该轨道对辅助配体的电子性质更敏感。计算表明,电荷从t BuC 6 H 3配体片段转移到主要过渡态的中央杂环中,这说明了烷基或氢化物配体的σ供体特性对性能的调节。
更新日期:2017-08-24
中文翻译:

的合成,结构,和发光的属性(C ∧ Ñ ∧ C)金(III)配合物的烷基:光电发射能和C-H酸度之间的相关性
的反应(C ∧ Ñ PZ ∧ C)AUCL与C-H酸性化合物ħ 2 CR 1 - [R 2在碱的存在下容易地得到相应的烷基络合物(C ∧ Ñ PZ ∧ C)AuCHR 1 - [R 2(2A, R 1= R 2= CN;3a,R 1= R 2= C(O)Me;4,R 1= R 2= CO 2 Et;5,R 1= R 2= C(O)Ph;R 1= R 2= CO 2 Et 。在图6a中,R 1= H,R 2= C(O)Me)。类似吡啶钳形络合物2B,3B,和图6B同样地得到(C ∧ Ñ PZ ∧ C = 2,6-双(4'-吨BUC 6 ħ 3)2吡嗪的二价阴离子;Ç ∧ Ñ PY ∧ C = 2 ,6-双(4' - t BuC 6 H 3)2吡啶二阴离子)。的(C反应∧ Ñ PZ ∧ C)AuOAc ˚F(7A)和(C∧ Ñ PY ∧ C)AuOAc ˚F(图7b)与MeMgI(C得到甲基络合物∧ Ñ PZ ∧ C)AuMe(8A()和C ∧ Ñ PY ∧ C)AuMe(8B分别地)。已经确定了2a,b,3a,6a和7a的晶体结构。新络合物的光物理特性以及那些以前报道的金的氢化物(C ∧ Ñ PZ ∧ C)AUH(AUH)的报告。较低的能量吸收和发射最大值按照能量序列2A < 3A < 4 < 5 < 6A < AUH ≈ 8A为吡嗪系列和图2b < 3B < 6B ≈ 8B为吡啶系列。这些值提供了与相应的辅助配体前体的p K a值的相关性。在DFT计算协议,排放被分配给3 IL(C ∧ Ñ ∧ C)/ 3 ILCT((C 6 H ^由HOMO和LUMO轨道控制的4 t Bu-4')→pz / py *)跃迁。LUMO位于杂环(py / pz)中,其位置与辅助配体相反,与HOMO相比,该轨道对辅助配体的电子性质更敏感。计算表明,电荷从t BuC 6 H 3配体片段转移到主要过渡态的中央杂环中,这说明了烷基或氢化物配体的σ供体特性对性能的调节。











































 京公网安备 11010802027423号
京公网安备 11010802027423号