Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2017-08-24 , DOI: 10.1016/j.bmcl.2017.08.050 Sherihan El-sayed , Kamel Metwally , Abdalla A. El-Shanawani , Lobna M. Abdel-Aziz , Ahmed A. El-Rashedy , Mahmoud E.S. Soliman , Luca Quattrini , Vito Coviello , Concettina la Motta
|
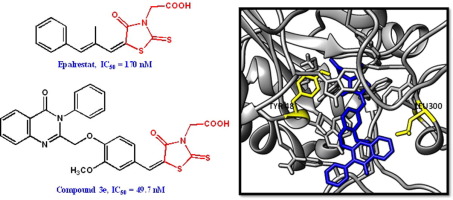
A series of quinazolinone-based rhodanine-3-acetic acids was synthesized and tested for in vitro aldose reductase inhibitory activity. All the target compounds displayed nanomolar activity against the target enzyme. Compounds 3a, 3b, and 3e exhibited almost 3-fold higher activity as compared to the only marketed reference drug epalrestat. Structure-activity relationship studies indicated that bulky substituents at the 3-phenyl ring of the quinazolinone moiety are generally not tolerated in the active site of the enzyme. Insertion of a methoxy group on the central benzylidene ring was found to have a variable effect on ALR-2 activity depending on the nature of peripheral quinazolinone ring substituents. Removal of the acetic acid moiety led to inactive or weakly active target compounds. Docking and molecular dynamic simulations of the most active rhodanine-3-acetic acid derivatives were also carried out, to provide the basis for further structure-guided design of novel inhibitors.
中文翻译:

喹唑啉酮基的Rhodananine-3-乙酸作为有效的醛糖还原酶抑制剂:合成,功能评估和分子模型研究
合成了一系列基于喹唑啉酮的若丹宁-3-乙酸,并测试了体外醛糖还原酶的抑制活性。所有目标化合物均显示出针对目标酶的纳摩尔活性。化合物3a,3b和3e与唯一市售的参比药物依帕司他相比,其活性几乎提高了三倍。结构-活性关系研究表明,在喹唑啉酮部分的3-苯基环上的庞大取代基通常不被酶的活性位点所容忍。发现甲氧基在中央亚苄基环上的插入对ALR-2活性具有可变的影响,这取决于外围的喹唑啉酮环取代基的性质。除去乙酸部分导致靶化合物失活或活性弱。还进行了对活性最强的Rhodananine-3-acetic acid衍生物的对接和分子动力学模拟,从而为新型抑制剂的进一步结构指导设计提供了基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号