Applied Catalysis A: General ( IF 4.7 ) Pub Date : 2017-08-24 , DOI: 10.1016/j.apcata.2017.08.025 Fengzhen Zhang , Chaohai Wei , Kaiyi Wu , Hongtao Zhou , Yun Hu , Sergei Preis
|
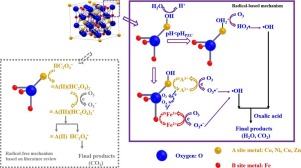
Spinel ferrites AFe2O4 (A = Co, Ni, Cu, and Zn) synthesized by citrate sol-gel method were tested for their catalytic performance in ozonation of aqueous oxalic acid. The objectives of this work include the evaluation of catalytic activity and ferrites stability in mineralization of oxalic acid together with exploring the activation mechanisms of ferrite catalysts in catalytic ozonation process. The results indicated that CoFe2O4 was the most active in catalyzing oxalic acid mineralization, removing 68.3% of TOC within 120 min in the reaction conditions of initial pH 2.3, oxalic acid concentration 5 mM, inlet ozone concentration 14 ± 1 mg L−1, catalyst dose 1.0 g L−1. All catalysts exhibited satisfactory composition stability in a batch experiment, although losing up to 4.67% of the catalyst mass in Fe3+ leaching from ZnFe2O4. H2-Temperature programmed reduction and cyclic voltammetry scan analysis revealed that these spinel ferrites had the potential reducibility and the electron donating ability to ozone molecule, respectively. Additionally, X-ray photoelectron spectroscopy showed that the surface metal ions’ valence state and the surface oxygen species played important roles in catalytic ozonation process. The reaction rate constant of oxalic acid mineralization were well fitted to non-linearly depend on variable metal valence and linearly depend on surface hydroxyl group density. The radical-based mechanistic reaction pathways involving A2+-A3+-A2+ cycle and positively charged surface hydroxyl groups were proposed.
中文翻译:

草酸臭氧氧化过程中铁氧体AFe 2 O 4(A = Co,Ni,Cu和Zn)催化性能的机理评估
测试了柠檬酸盐溶胶-凝胶法合成的尖晶石型铁氧体AFe 2 O 4(A = Co,Ni,Cu和Zn)在草酸水溶液臭氧化反应中的催化性能。这项工作的目的包括评估草酸在矿化过程中的催化活性和铁素体稳定性,以及探索催化臭氧化过程中铁素体催化剂的活化机理。结果表明,CoFe 2 O 4催化草酸矿化的活性最高,在初始pH为2.3,草酸浓度为5 mM,入口臭氧浓度为14±1 mg L-的反应条件下,在120分钟内去除了68.3%的TOC 。 1,催化剂剂量1.0 g L -1。尽管在从ZnFe 2 O 4浸出的Fe 3+中损失了高达4.67%的催化剂质量,但所有催化剂在分批实验中均表现出令人满意的组成稳定性。高2-温度程序化还原和循环伏安扫描分析表明,这些尖晶石铁氧体分别具有对臭氧分子的电势还原能力和给电子能力。另外,X射线光电子能谱表明,表面金属离子的化合价态和表面氧的种类在催化臭氧化过程中起着重要的作用。草酸矿化的反应速率常数非常适合非线性地取决于可变的金属化合价并且线性地取决于表面羟基的密度。提出了基于自由基的机械反应途径,涉及A 2+ -A 3+ -A 2+循环和带正电荷的表面羟基。











































 京公网安备 11010802027423号
京公网安备 11010802027423号