Tetrahedron ( IF 2.1 ) Pub Date : 2017-08-24 , DOI: 10.1016/j.tet.2017.08.038 Đani Škalamera , Jelena Veljković , Lucija Ptiček , Matija Sambol , Kata Mlinarić-Majerski , Nikola Basarić
|
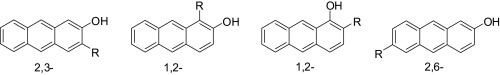
We have developed synthetic pathways toward differently substituted hydroxyanthracenes (anthrols) with the aim to investigate their photochemical reactivity in dehydration reactions. Although the syntheses of anthracenes substituted at positions 9,10 are well known, reports for the synthesis of anthracenes with different substitution patterns are scarce. Herein we review known and report novel synthetic pathways toward anthrols with substituents at 1,2-, 2,3-, and 2,6- positions. We present two synthetic approaches: (i) building of the anthracene tricyclic fused ring system from the appropriate benzene derivatives, and (ii) reduction of the corresponding anthraquinones.
Reduction of 2-hydroxyanthracene-1-carbaldehyde to the corresponding alcohol yields rather unexpected 1,1′-methylenedianthracen-2-ol, whose proposed mechanism of formation is supported by experimental observations and calculations.
中文翻译:

不对称双取代蒽的合成
我们已经开发出了合成途径通往不同取代的羟基蒽(蒽),目的是研究它们在脱水反应中的光化学反应性。尽管在9,10位被取代的蒽的合成是众所周知的,但是关于具有不同取代模式的蒽的合成的报道却很少。在本文中,我们综述了已知的并且报道了在1,2-,2,3-和2,6-位具有取代基的蒽的新颖合成途径。我们提出了两种合成方法:(i)由适当的苯衍生物构建蒽三环稠环系统,以及(ii)还原相应的蒽醌。
将2-羟基蒽-1-甲醛还原成相应的醇会产生出乎意料的1,1'-亚甲基蒽-2-醇,其拟议的形成机理得到了实验观察和计算的支持。











































 京公网安备 11010802027423号
京公网安备 11010802027423号