Molecular Cell ( IF 14.5 ) Pub Date : 2017-08-10 , DOI: 10.1016/j.molcel.2017.07.004 Javaid Y. Bhat , Goran Miličić , Gabriel Thieulin-Pardo , Andreas Bracher , Andrew Maxwell , Susanne Ciniawsky , Oliver Mueller-Cajar , John R. Engen , F. Ulrich Hartl , Petra Wendler , Manajit Hayer-Hartl
|
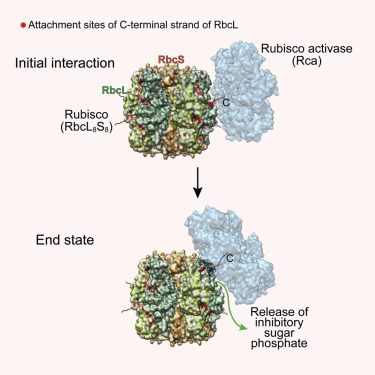
How AAA+ chaperones conformationally remodel specific target proteins in an ATP-dependent manner is not well understood. Here, we investigated the mechanism of the AAA+ protein Rubisco activase (Rca) in metabolic repair of the photosynthetic enzyme Rubisco, a complex of eight large (RbcL) and eight small (RbcS) subunits containing eight catalytic sites. Rubisco is prone to inhibition by tight-binding sugar phosphates, whose removal is catalyzed by Rca. We engineered a stable Rca hexamer ring and analyzed its functional interaction with Rubisco. Hydrogen/deuterium exchange and chemical crosslinking showed that Rca structurally destabilizes elements of the Rubisco active site with remarkable selectivity. Cryo-electron microscopy revealed that Rca docks onto Rubisco over one active site at a time, positioning the C-terminal strand of RbcL, which stabilizes the catalytic center, for access to the Rca hexamer pore. The pulling force of Rca is fine-tuned to avoid global destabilization and allow for precise enzyme repair.
中文翻译:

AAA +分子伴侣Rubisco活化酶修复酶的机理
AAA +分子构象如何以ATP依赖性方式重塑特定的靶蛋白尚不清楚。在这里,我们研究了AAA +蛋白Rubisco激活酶(Rca)在光合作用酶Rubisco的代谢修复中的机制,该光合作用是8个大(RbcL)和8个小(RbcS)亚基的复合物,其中包含8个催化位点。Rubisco易于受到紧密结合的糖磷酸酯的抑制,而Rca催化磷酸的去除。我们设计了一个稳定的Rca六聚环,并分析了其与Rubisco的功能相互作用。氢/氘交换和化学交联表明,Rca具有显着的选择性,使Rubisco活性位点的元素不稳定。低温电子显微镜显示,Rca一次在一个活性位点停靠在Rubisco上,将RbcL的C端链定位,它可以稳定催化中心,以便进入Rca六聚体孔。Rca的拉力经过微调,可以避免整体不稳定,并可以进行精确的酶修复。











































 京公网安备 11010802027423号
京公网安备 11010802027423号