Molecular Cell ( IF 14.5 ) Pub Date : 2017-07-06 , DOI: 10.1016/j.molcel.2017.06.011 Vivien Ya-Fan Wang , Yidan Li , Daniel Kim , Xiangyang Zhong , Qian Du , Majid Ghassemian , Gourisankar Ghosh
|
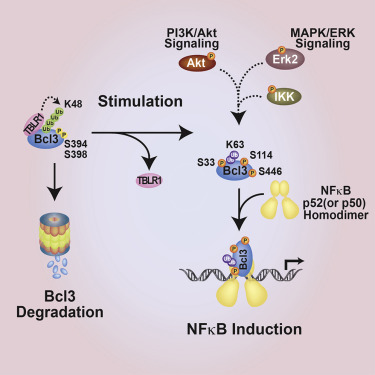
Unlike prototypical IκB proteins, which are inhibitors of NF-κB RelA, cRel, and RelB dimers, the atypical IκB protein Bcl3 is primarily a transcriptional coregulator of p52 and p50 homodimers. Bcl3 exists as phospho-protein in many cancer cells. Unphosphorylated Bcl3 acts as a classical IκB-like inhibitor and removes p50 and p52 from bound DNA. Neither the phosphorylation site(s) nor the kinase(s) phosphorylating Bcl3 is known. Here we show that Akt, Erk2, and IKK1/2 phosphorylate Bcl3. Phosphorylation of Ser33 by Akt induces switching of K48 ubiquitination to K63 ubiquitination and thus promotes nuclear localization and stabilization of Bcl3. Phosphorylation by Erk2 and IKK1/2 of Ser114 and Ser446 converts Bcl3 into a transcriptional coregulator by facilitating its recruitment to DNA. Cells expressing the S114A/S446A mutant have cellular proliferation and migration defects. This work links Akt and MAPK pathways to NF-κB through Bcl3 and provides mechanistic insight into how Bcl3 functions as an oncoprotein through collaboration with IKK1/2, Akt, and Erk2.
中文翻译:

Akt,Erk2和IKK的Bcl3磷酸化是其转录活性所必需的。
与典型的IκB蛋白是NF-κBRelA,cRel和RelB二聚体的抑制剂不同,非典型IκB蛋白Bcl3主要是p52和p50同型二聚体的转录共调节子。Bcl3作为磷酸蛋白存在于许多癌细胞中。未磷酸化的Bcl3充当经典的IκB样抑制剂,并从结合的DNA中去除p50和p52。磷酸化位点或磷酸化Bcl3的激酶都不是已知的。在这里,我们显示Akt,Erk2和IKK1 / 2磷酸化Bcl3。Akt对Ser33的磷酸化诱导将K48泛素化切换为K63泛素化,从而促进Bcl3的核定位和稳定化。Ser114和Ser446的Erk2和IKK1 / 2进行的磷酸化通过促进Bcl3募集到DNA而将Bcl3转化为转录共调节因子。表达S114A / S446A突变体的细胞具有细胞增殖和迁移缺陷。这项工作通过Bcl3将Akt和MAPK途径与NF-κB连接起来,并通过与IKK1 / 2,Akt和Erk2的合作提供了有关Bcl3如何作为癌蛋白起作用的机制的见解。









































 京公网安备 11010802027423号
京公网安备 11010802027423号