当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Validation of Dissolution Testing with Biorelevant Media: An OrBiTo Study
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2017-08-23 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00198 James Mann 1 , Jennifer Dressman 2 , Karin Rosenblatt 3 , Lee Ashworth 1 , Uwe Muenster 4 , Kerstin Frank 5 , Paul Hutchins 6 , James Williams 7 , Lukas Klumpp 2 , Kristina Wielockx 8 , Philippe Berben 9 , Patrick Augustijns 9 , Rene Holm 10 , Michael Hofmann 11 , Sanjaykumar Patel 12 , Stefania Beato 13 , Krista Ojala 14 , Irena Tomaszewska 15 , Jean-Luc Bruel 16 , James Butler 7
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2017-08-23 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00198 James Mann 1 , Jennifer Dressman 2 , Karin Rosenblatt 3 , Lee Ashworth 1 , Uwe Muenster 4 , Kerstin Frank 5 , Paul Hutchins 6 , James Williams 7 , Lukas Klumpp 2 , Kristina Wielockx 8 , Philippe Berben 9 , Patrick Augustijns 9 , Rene Holm 10 , Michael Hofmann 11 , Sanjaykumar Patel 12 , Stefania Beato 13 , Krista Ojala 14 , Irena Tomaszewska 15 , Jean-Luc Bruel 16 , James Butler 7
Affiliation
|
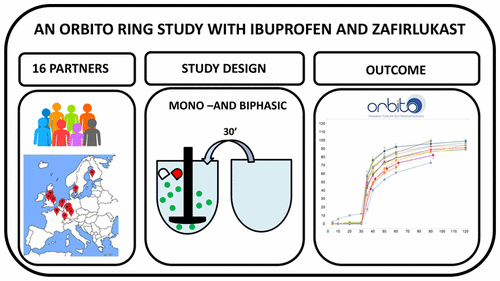
Dissolution testing with biorelevant media has become widespread in the pharmaceutical industry as a means of better understanding how drugs and formulations behave in the gastrointestinal tract. Until now, however, there have been few attempts to gauge the reproducibility of results obtained with these methods. The aim of this study was to determine the interlaboratory reproducibility of biorelevant dissolution testing, using the paddle apparatus (USP 2). Thirteen industrial and three academic laboratories participated in this study. All laboratories were provided with standard protocols for running the tests: dissolution in FaSSGF to simulate release in the stomach, dissolution in a single intestinal medium, FaSSIF, to simulate release in the small intestine, and a “transfer” (two-stage) protocol to simulate the concentration profile when conditions are changed from the gastric to the intestinal environment. The test products chosen were commercially available ibuprofen tablets and zafirlukast tablets. The biorelevant dissolution tests showed a high degree of reproducibility among the participating laboratories, even though several different batches of the commercially available medium preparation powder were used. Likewise, results were almost identicalbetween the commercial biorelevant media and those produced in-house. Comparing results to previous ring studies, including those performed with USP calibrator tablets or commercially available pharmaceutical products in a single medium, the results for the biorelevant studies were highly reproducible on an interlaboratory basis. Interlaboratory reproducibility with the two-stage test was also acceptable, although the variability was somewhat greater than with the single medium tests. Biorelevant dissolution testing is highly reproducible among laboratories and can be relied upon for cross-laboratory comparisons.
中文翻译:

生物相关介质的溶出度测试验证:OrBiTo研究
使用生物相关介质的溶出度测试已成为制药行业中的一种手段,可以更好地了解药物和制剂在胃肠道中的行为。但是,到目前为止,很少有人尝试评估使用这些方法获得的结果的可重复性。这项研究的目的是使用桨装置(USP 2)确定生物相关溶出度试验在实验室间的可重复性。13个工业实验室和3个学术实验室参加了这项研究。为所有实验室提供了运行测试的标准方案:在FaSSGF中溶解以模拟在胃中的释放,在单个肠介质FaSSIF中溶解以模拟在小肠中的释放,和“转移”(两阶段)协议,可在条件从胃部变为肠道环境时模拟浓度曲线。选择的测试产品是可商购的布洛芬片剂和扎鲁司特片剂。与生物有关的溶出度测试表明,即使使用了几批不同的市售培养基制剂粉末,参与实验室也具有高度的可重复性。同样,商业生物相关介质与内部产生的介质之间的结果几乎相同。与以前的环形研究(包括在单一介质中使用USP校准片或市售药品进行的研究)的结果进行比较,在实验室间的基础上,与生物相关的研究结果具有很高的可重复性。两阶段试验的实验室间再现性也可以接受,尽管变异性比单一培养基试验更大。与生物有关的溶出度测试在实验室之间具有很高的可重复性,可以作为跨实验室比较的依据。
更新日期:2017-08-23
中文翻译:

生物相关介质的溶出度测试验证:OrBiTo研究
使用生物相关介质的溶出度测试已成为制药行业中的一种手段,可以更好地了解药物和制剂在胃肠道中的行为。但是,到目前为止,很少有人尝试评估使用这些方法获得的结果的可重复性。这项研究的目的是使用桨装置(USP 2)确定生物相关溶出度试验在实验室间的可重复性。13个工业实验室和3个学术实验室参加了这项研究。为所有实验室提供了运行测试的标准方案:在FaSSGF中溶解以模拟在胃中的释放,在单个肠介质FaSSIF中溶解以模拟在小肠中的释放,和“转移”(两阶段)协议,可在条件从胃部变为肠道环境时模拟浓度曲线。选择的测试产品是可商购的布洛芬片剂和扎鲁司特片剂。与生物有关的溶出度测试表明,即使使用了几批不同的市售培养基制剂粉末,参与实验室也具有高度的可重复性。同样,商业生物相关介质与内部产生的介质之间的结果几乎相同。与以前的环形研究(包括在单一介质中使用USP校准片或市售药品进行的研究)的结果进行比较,在实验室间的基础上,与生物相关的研究结果具有很高的可重复性。两阶段试验的实验室间再现性也可以接受,尽管变异性比单一培养基试验更大。与生物有关的溶出度测试在实验室之间具有很高的可重复性,可以作为跨实验室比较的依据。



























 京公网安备 11010802027423号
京公网安备 11010802027423号