当前位置:
X-MOL 学术
›
Mater. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct amination of the antiaromatic NiII norcorrole†
Materials Chemistry Frontiers ( IF 6.0 ) Pub Date : 2017-05-10 00:00:00 , DOI: 10.1039/c7qm00176b Takuya Yoshida 1, 2, 3, 4, 5 , Hiroshi Shinokubo 1, 2, 3, 4, 5
Materials Chemistry Frontiers ( IF 6.0 ) Pub Date : 2017-05-10 00:00:00 , DOI: 10.1039/c7qm00176b Takuya Yoshida 1, 2, 3, 4, 5 , Hiroshi Shinokubo 1, 2, 3, 4, 5
Affiliation
|
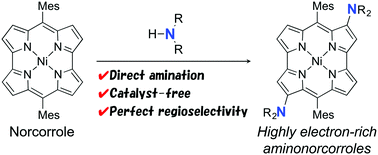
We have discovered a facile amination reaction of NiII dimesitylnorcorrole, which is an antiaromatic porphyrinoid with a 16π-electron conjugation system. The amination reaction proceeded at the β-positions of the norcorrole with high regioselectivity upon treatment with various amines used as solvents. The reaction requires neither catalysts nor pre-functionalization of the norcorrole. The optical and electrochemical properties of the amino norcorroles were substantially modulated by electron-donating nitrogen atoms. According to theoretical studies, the regioselectivity of amination can be accounted for by molecular orbitals of the norcorrole.
中文翻译:

抗芳香族Ni II正戊基甲酸酯的直接胺化†
我们已经发现Ni II二聚体inorcorrole的胺化反应很容易,这是一种具有16π电子共轭体系的抗芳族卟啉类化合物。用各种用作溶剂的胺处理后,胺化反应在正戊醇的β-位以高区域选择性进行。该反应既不需要催化剂也不需要正戊烷的预官能化。氨基去甲酚的光学和电化学性质基本上受供电子氮原子的调节。根据理论研究,胺化的区域选择性可以通过正戊烯醇的分子轨道来解释。
更新日期:2017-05-10
中文翻译:

抗芳香族Ni II正戊基甲酸酯的直接胺化†
我们已经发现Ni II二聚体inorcorrole的胺化反应很容易,这是一种具有16π电子共轭体系的抗芳族卟啉类化合物。用各种用作溶剂的胺处理后,胺化反应在正戊醇的β-位以高区域选择性进行。该反应既不需要催化剂也不需要正戊烷的预官能化。氨基去甲酚的光学和电化学性质基本上受供电子氮原子的调节。根据理论研究,胺化的区域选择性可以通过正戊烯醇的分子轨道来解释。











































 京公网安备 11010802027423号
京公网安备 11010802027423号