当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient trifluoromethylation via the cyclopropanation of allenes and subsequent C–C bond cleavage
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-06-23 00:00:00 , DOI: 10.1039/c7qo00419b Yang Tang 1, 2, 3, 4, 5 , Qiong Yu 5, 6, 7, 8 , Shengming Ma 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-06-23 00:00:00 , DOI: 10.1039/c7qo00419b Yang Tang 1, 2, 3, 4, 5 , Qiong Yu 5, 6, 7, 8 , Shengming Ma 1, 2, 3, 4, 5
Affiliation
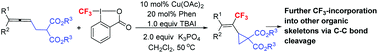
|
As we know, the incorporation of a trifluoromethyl group into organic molecules may significantly alter their physical and biological properties due to the high electronegativity, lipophilicity, and excellent metabolic stability of the trifluoromethyl substituent. Thus, an efficient method for the introduction of the trifluoromethyl group is of high current interest. On the other hand, vinylic cyclopropanes are a class of strained compounds capable of undergoing ring-opening reaction with other molecules. Here, CF3-substituted vinylic cyclopropanes have been highly selectively formed by a copper-catalyzed cyclic trifluoromethylation of (4,4-disubstituted-2,3-butadienyl)malonates with Togni's reagent II, in which the trifluoromethyl group was installed at the middle carbon of the allene unit by applying 1,10-phenanthroline as the ligand. Such unique cyclopropanes successfully bring the trifluoromethyl group to other useful organic skeletons by the selective cleavage of C–C bonds with an exclusive diastereoselectivity. Based on the mechanistic studies, an allene radical addition, oxidation, and allylic substitution pathway has been proposed.
中文翻译:

通过丙二烯的环丙烷化和随后的C–C键裂解实现有效的三氟甲基化
众所周知,由于三氟甲基取代基的高电负性,亲脂性和优异的代谢稳定性,将三氟甲基掺入有机分子可能会显着改变其物理和生物学特性。因此,引入三氟甲基的有效方法在当前受到广泛关注。另一方面,乙烯基环丙烷是一类能够与其他分子发生开环反应的应变化合物。CF 3(4,4-二取代的2,3-丁二烯基)丙二酸酯的铜催化的环状三氟甲基化用Togni的试剂II高度选择性地形成了取代的乙烯基环丙烷,其中三氟甲基被安装在丙二烯的中间碳上通过应用1,10-菲咯啉作为配体。这种独特的环丙烷可通过独有的非对映选择性选择性裂解C–C键,成功地将三氟甲基带到其他有用的有机骨架上。在机理研究的基础上,提出了烯丙基自由基的加成,氧化和烯丙基取代的途径。
更新日期:2017-08-23
中文翻译:

通过丙二烯的环丙烷化和随后的C–C键裂解实现有效的三氟甲基化
众所周知,由于三氟甲基取代基的高电负性,亲脂性和优异的代谢稳定性,将三氟甲基掺入有机分子可能会显着改变其物理和生物学特性。因此,引入三氟甲基的有效方法在当前受到广泛关注。另一方面,乙烯基环丙烷是一类能够与其他分子发生开环反应的应变化合物。CF 3(4,4-二取代的2,3-丁二烯基)丙二酸酯的铜催化的环状三氟甲基化用Togni的试剂II高度选择性地形成了取代的乙烯基环丙烷,其中三氟甲基被安装在丙二烯的中间碳上通过应用1,10-菲咯啉作为配体。这种独特的环丙烷可通过独有的非对映选择性选择性裂解C–C键,成功地将三氟甲基带到其他有用的有机骨架上。在机理研究的基础上,提出了烯丙基自由基的加成,氧化和烯丙基取代的途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号