当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
meta-Selective C–H difluoromethylation of various arenes with a versatile ruthenium catalyst
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-06-19 00:00:00 , DOI: 10.1039/c7qo00449d C. C. Yuan 1, 2, 3, 4, 5 , X. L. Chen 1, 2, 3, 4, 5 , J. Y. Zhang 4, 5, 6, 7, 8 , Y. S. Zhao 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-06-19 00:00:00 , DOI: 10.1039/c7qo00449d C. C. Yuan 1, 2, 3, 4, 5 , X. L. Chen 1, 2, 3, 4, 5 , J. Y. Zhang 4, 5, 6, 7, 8 , Y. S. Zhao 1, 2, 3, 4, 5
Affiliation
|
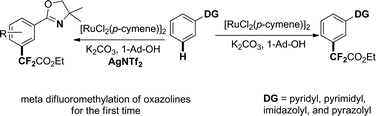
A ruthenium-enabled meta-selective C–H difluoromethylation of arenes has been developed. Various arenes bearing pyridyl, pyrazolyl, imidazolyl, or pyrimidyl directing groups, or removable oxazoline directing groups, were tolerated in this meta-selective C–H difluoromethylation, affording the difluoromethylated products in moderate to good yields. The difluoromethyl group could be selectively installed into several drug molecules and bioactive compounds in one step, highlighting the synthetic utility and importance of this new method.
中文翻译:

多功能钌催化剂对各种芳烃的间位选择性C–H二氟甲基化
开发了钌使能的芳烃间位选择性C–H二氟甲基化反应。轴承吡啶基,吡唑基,咪唑基,或嘧啶基定位基团,或可移动的引导恶唑啉基团的各种芳烃,在此进行了耐受元-选择性C-H二氟甲基,得difluoromethylated产品在中度至良好的产率。可以一步将二氟甲基选择性地安装到几个药物分子和生物活性化合物中,从而突出了这种新方法的合成实用性和重要性。
更新日期:2017-08-23
中文翻译:

多功能钌催化剂对各种芳烃的间位选择性C–H二氟甲基化
开发了钌使能的芳烃间位选择性C–H二氟甲基化反应。轴承吡啶基,吡唑基,咪唑基,或嘧啶基定位基团,或可移动的引导恶唑啉基团的各种芳烃,在此进行了耐受元-选择性C-H二氟甲基,得difluoromethylated产品在中度至良好的产率。可以一步将二氟甲基选择性地安装到几个药物分子和生物活性化合物中,从而突出了这种新方法的合成实用性和重要性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号