当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electroreduction of CO2 Catalyzed by a Heterogenized Zn–Porphyrin Complex with a Redox-Innocent Metal Center
ACS Central Science ( IF 12.7 ) Pub Date : 2017-07-26 00:00:00 , DOI: 10.1021/acscentsci.7b00160 Yueshen Wu 1, 2 , Jianbing Jiang 1, 2 , Zhe Weng 1, 2 , Maoyu Wang 3 , Daniël L. J. Broere 1 , Yiren Zhong 1, 2 , Gary W. Brudvig 1, 2 , Zhenxing Feng 3 , Hailiang Wang 1, 2
ACS Central Science ( IF 12.7 ) Pub Date : 2017-07-26 00:00:00 , DOI: 10.1021/acscentsci.7b00160 Yueshen Wu 1, 2 , Jianbing Jiang 1, 2 , Zhe Weng 1, 2 , Maoyu Wang 3 , Daniël L. J. Broere 1 , Yiren Zhong 1, 2 , Gary W. Brudvig 1, 2 , Zhenxing Feng 3 , Hailiang Wang 1, 2
Affiliation
|
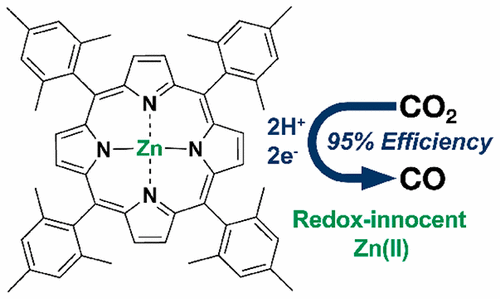
Transition-metal-based molecular complexes are a class of catalyst materials for electrochemical CO2 reduction to CO that can be rationally designed to deliver high catalytic performance. One common mechanistic feature of these electrocatalysts developed thus far is an electrogenerated reduced metal center associated with catalytic CO2 reduction. Here we report a heterogenized zinc–porphyrin complex (zinc(II) 5,10,15,20-tetramesitylporphyrin) as an electrocatalyst that delivers a turnover frequency as high as 14.4 site–1 s–1 and a Faradaic efficiency as high as 95% for CO2 electroreduction to CO at −1.7 V vs the standard hydrogen electrode in an organic/water mixed electrolyte. While the Zn center is critical to the observed catalysis, in situ and operando X-ray absorption spectroscopic studies reveal that it is redox-innocent throughout the potential range. Cyclic voltammetry indicates that the porphyrin ligand may act as a redox mediator. Chemical reduction of the zinc–porphyrin complex further confirms that the reduction is ligand-based and the reduced species can react with CO2. This represents the first example of a transition-metal complex for CO2 electroreduction catalysis with its metal center being redox-innocent under working conditions.
中文翻译:

异质性氧化锌-卟啉配合物与氧化纯金属中心催化CO 2的电还原
基于过渡金属的分子络合物是一类用于将CO 2电化学还原为CO的催化剂材料,可以合理设计以提供高催化性能。迄今为止,这些电催化剂的一个共同的机械特征是与催化的CO 2还原有关的电还原金属中心。在这里,我们报告了一种异质化的锌-卟啉配合物(锌(II)5,10,15,20-四甲苯基卟啉)作为电催化剂,其周转频率高达14.4位–1 s –1,法拉第效率高达95 CO 2的百分比在有机/水混合电解质中,相对于标准氢电极,在-1.7 V下电还原为CO。尽管锌中心对观察到的催化作用至关重要,但原位和操作X射线吸收光谱研究表明,它在整个电位范围内都是无毒的。循环伏安法表明卟啉配体可以充当氧化还原介体。锌-卟啉配合物的化学还原进一步证实了该还原是基于配体的,并且还原的物种可以与CO 2反应。这代表了用于CO 2电还原催化的过渡金属配合物的第一个例子,其金属中心在工作条件下为无毒氧化物。
更新日期:2017-08-23
中文翻译:

异质性氧化锌-卟啉配合物与氧化纯金属中心催化CO 2的电还原
基于过渡金属的分子络合物是一类用于将CO 2电化学还原为CO的催化剂材料,可以合理设计以提供高催化性能。迄今为止,这些电催化剂的一个共同的机械特征是与催化的CO 2还原有关的电还原金属中心。在这里,我们报告了一种异质化的锌-卟啉配合物(锌(II)5,10,15,20-四甲苯基卟啉)作为电催化剂,其周转频率高达14.4位–1 s –1,法拉第效率高达95 CO 2的百分比在有机/水混合电解质中,相对于标准氢电极,在-1.7 V下电还原为CO。尽管锌中心对观察到的催化作用至关重要,但原位和操作X射线吸收光谱研究表明,它在整个电位范围内都是无毒的。循环伏安法表明卟啉配体可以充当氧化还原介体。锌-卟啉配合物的化学还原进一步证实了该还原是基于配体的,并且还原的物种可以与CO 2反应。这代表了用于CO 2电还原催化的过渡金属配合物的第一个例子,其金属中心在工作条件下为无毒氧化物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号