当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Membrane Binding of Recoverin: From Mechanistic Understanding to Biological Functionality
ACS Central Science ( IF 12.7 ) Pub Date : 2017-07-24 00:00:00 , DOI: 10.1021/acscentsci.7b00210 Štěpán Timr 1 , Roman Pleskot 1, 2 , Jan Kadlec 1 , Miriam Kohagen 1, 3 , Aniket Magarkar 1, 4 , Pavel Jungwirth 1, 5
ACS Central Science ( IF 12.7 ) Pub Date : 2017-07-24 00:00:00 , DOI: 10.1021/acscentsci.7b00210 Štěpán Timr 1 , Roman Pleskot 1, 2 , Jan Kadlec 1 , Miriam Kohagen 1, 3 , Aniket Magarkar 1, 4 , Pavel Jungwirth 1, 5
Affiliation
|
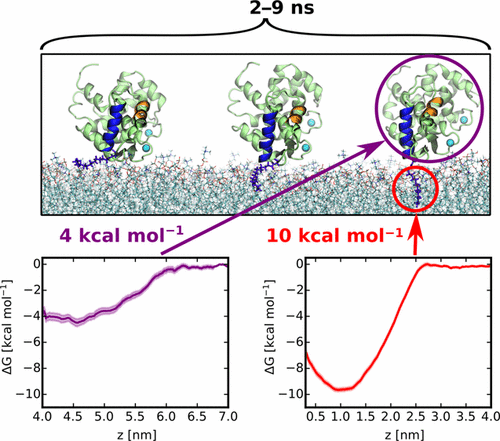
Recoverin is a neuronal calcium sensor involved in vision adaptation that reversibly associates with cellular membranes via its calcium-activated myristoyl switch. While experimental evidence shows that the myristoyl group significantly enhances membrane affinity of this protein, molecular details of the binding process are still under debate. Here, we present results of extensive molecular dynamics simulations of recoverin in the proximity of a phospholipid bilayer. We capture multiple events of spontaneous membrane insertion of the myristoyl moiety and confirm its critical role in the membrane binding. Moreover, we observe that the binding strongly depends on the conformation of the N-terminal domain. We propose that a suitable conformation of the N-terminal domain can be stabilized by the disordered C-terminal segment or by binding of the target enzyme, i.e., rhodopsin kinase. Finally, we find that the presence of negatively charged lipids in the bilayer stabilizes a physiologically functional orientation of the membrane-bound recoverin.
中文翻译:

恢复蛋白的膜结合:从机制的理解到生物学功能。
Recoverin是一种涉及视力适应的神经元钙传感器,通过其钙激活的肉豆蔻酰开关可逆地与细胞膜结合。尽管实验证据表明肉豆蔻酰基可显着增强该蛋白的膜亲和力,但结合过程的分子细节仍在争论中。在这里,我们介绍磷脂双层附近的回收蛋白的广泛分子动力学模拟的结果。我们捕获肉豆蔻酰基部分自发膜插入的多个事件,并确认其在膜结合中的关键作用。而且,我们观察到结合强烈地依赖于N-末端结构域的构象。我们提出,可以通过无序的C末端区段或通过结合靶酶即视紫红质激酶来稳定N末端结构域的合适构象。最后,我们发现双层中带负电荷的脂质的存在稳定了膜结合的coverin的生理功能方向。
更新日期:2017-08-23
中文翻译:

恢复蛋白的膜结合:从机制的理解到生物学功能。
Recoverin是一种涉及视力适应的神经元钙传感器,通过其钙激活的肉豆蔻酰开关可逆地与细胞膜结合。尽管实验证据表明肉豆蔻酰基可显着增强该蛋白的膜亲和力,但结合过程的分子细节仍在争论中。在这里,我们介绍磷脂双层附近的回收蛋白的广泛分子动力学模拟的结果。我们捕获肉豆蔻酰基部分自发膜插入的多个事件,并确认其在膜结合中的关键作用。而且,我们观察到结合强烈地依赖于N-末端结构域的构象。我们提出,可以通过无序的C末端区段或通过结合靶酶即视紫红质激酶来稳定N末端结构域的合适构象。最后,我们发现双层中带负电荷的脂质的存在稳定了膜结合的coverin的生理功能方向。











































 京公网安备 11010802027423号
京公网安备 11010802027423号