当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Hydroxylation of Polyethylenes
ACS Central Science ( IF 12.7 ) Pub Date : 2017-08-09 00:00:00 , DOI: 10.1021/acscentsci.7b00255 Ala Bunescu 1 , Sunwoo Lee 1, 2 , Qian Li 1 , John F. Hartwig 1
ACS Central Science ( IF 12.7 ) Pub Date : 2017-08-09 00:00:00 , DOI: 10.1021/acscentsci.7b00255 Ala Bunescu 1 , Sunwoo Lee 1, 2 , Qian Li 1 , John F. Hartwig 1
Affiliation
|
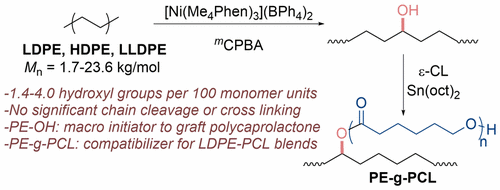
Polyolefins account for 60% of global plastic consumption, but many potential applications of polyolefins require that their properties, such as compatibility with polar polymers, adhesion, gas permeability, and surface wetting, be improved. A strategy to overcome these deficiencies would involve the introduction of polar functionalities onto the polymer chain. Here, we describe the Ni-catalyzed hydroxylation of polyethylenes (LDPE, HDPE, and LLDPE) in the presence of mCPBA as an oxidant. Studies with cycloalkanes and pure, long-chain alkanes were conducted to assess precisely the selectivity of the reaction and the degree to which potential C–C bond cleavage of a radical intermediate occurs. Among the nickel catalysts we tested, [Ni(Me4Phen)3](BPh4)2 (Me4Phen = 3,4,7,8,-tetramethyl-1,10-phenanthroline) reacted with the highest turnover number (TON) for hydroxylation of cyclohexane and the highest selectivity for the formation of cyclohexanol over cyclohexanone (TON, 5560; cyclohexanol/(cyclohexanone + ε-caprolactone) ratio, 10.5). The oxidation of n-octadecane occurred at the secondary C–H bonds with 15.5:1 selectivity for formation of an alcohol over a ketone and 660 TON. Consistent with these data, the hydroxylation of various polyethylene materials by the combination of [Ni(Me4Phen)3](BPh4)2 and mCPBA led to the introduction of 2.0 to 5.5 functional groups (alcohol, ketone, alkyl chloride) per 100 monomer units with up to 88% selectivity for formation of alcohols over ketones or chloride. In contrast to more classical radical functionalizations of polyethylene, this catalytic process occurred without significant modification of the molecular weight of the polymer that would result from chain cleavage or cross-linking. Thus, the resulting materials are new compositions in which hydroxyl groups are located along the main chain of commercial, high molecular weight LDPE, HDPE, and LLDPE materials. These hydroxylated polyethylenes have improved wetting properties and serve as macroinitiators to synthesize graft polycaprolactones that compatibilize polyethylene–polycaprolactone blends.
中文翻译:

聚乙烯的催化羟基化
聚烯烃占全球塑料消耗量的60%,但是聚烯烃的许多潜在应用要求改善其性能,例如与极性聚合物的相容性,粘合性,透气性和表面润湿性。克服这些缺陷的策略将涉及在聚合物链上引入极性官能团。在这里,我们描述了在m CPBA作为氧化剂存在下聚乙烯(LDPE,HDPE和LLDPE)的Ni催化羟基化反应。用环烷烃和纯长链烷烃进行的研究可以精确评估反应的选择性以及发生自由基中间体发生C–C键断裂的程度。在我们测试的镍催化剂中,[Ni(Me 4 Phen)3 ](BPh4)2(Me 4 Phen = 3,4,7,8,-tetramethyl-1,10-phenothroline)与环己烷羟化反应的最高周转数(TON)和形成环己醇的选择性最高( TON,5560;环己醇/(环己酮+ε-己内酯)比率,10.5)。正十八烷的氧化发生在二级CH键上,在酮和660 TON上形成醇的选择性为15.5:1。与这些数据一致,[Ni(Me 4 Phen)3 ](BPh 4)2和m的组合使各种聚乙烯材料羟基化CPBA导致每100个单体单元引入2.0至5.5个官能团(醇,酮,烷基氯),与酮或氯相比,形成醇的选择性高达88%。与聚乙烯的更经典的自由基官能化相反,该催化过程的发生没有明显改变由链断裂或交联引起的聚合物分子量。因此,所得材料是新的组合物,其中羟基沿市售的高分子量LDPE,HDPE和LLDPE材料的主链分布。这些羟基化的聚乙烯具有改善的润湿性能,并可以作为大分子引发剂来合成接枝的聚己内酯,从而使聚乙烯与聚己内酯的混合物相容。
更新日期:2017-08-23
中文翻译:

聚乙烯的催化羟基化
聚烯烃占全球塑料消耗量的60%,但是聚烯烃的许多潜在应用要求改善其性能,例如与极性聚合物的相容性,粘合性,透气性和表面润湿性。克服这些缺陷的策略将涉及在聚合物链上引入极性官能团。在这里,我们描述了在m CPBA作为氧化剂存在下聚乙烯(LDPE,HDPE和LLDPE)的Ni催化羟基化反应。用环烷烃和纯长链烷烃进行的研究可以精确评估反应的选择性以及发生自由基中间体发生C–C键断裂的程度。在我们测试的镍催化剂中,[Ni(Me 4 Phen)3 ](BPh4)2(Me 4 Phen = 3,4,7,8,-tetramethyl-1,10-phenothroline)与环己烷羟化反应的最高周转数(TON)和形成环己醇的选择性最高( TON,5560;环己醇/(环己酮+ε-己内酯)比率,10.5)。正十八烷的氧化发生在二级CH键上,在酮和660 TON上形成醇的选择性为15.5:1。与这些数据一致,[Ni(Me 4 Phen)3 ](BPh 4)2和m的组合使各种聚乙烯材料羟基化CPBA导致每100个单体单元引入2.0至5.5个官能团(醇,酮,烷基氯),与酮或氯相比,形成醇的选择性高达88%。与聚乙烯的更经典的自由基官能化相反,该催化过程的发生没有明显改变由链断裂或交联引起的聚合物分子量。因此,所得材料是新的组合物,其中羟基沿市售的高分子量LDPE,HDPE和LLDPE材料的主链分布。这些羟基化的聚乙烯具有改善的润湿性能,并可以作为大分子引发剂来合成接枝的聚己内酯,从而使聚乙烯与聚己内酯的混合物相容。









































 京公网安备 11010802027423号
京公网安备 11010802027423号