当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Influence of Physiological Gastrointestinal Surfactant Ratio on the Equilibrium Solubility of BCS Class II Drugs Investigated Using a Four Component Mixture Design
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-08-22 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00354 Zhou Zhou 1 , Claire Dunn 1 , Ibrahim Khadra 1 , Clive G. Wilson 1 , Gavin W. Halbert 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-08-22 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00354 Zhou Zhou 1 , Claire Dunn 1 , Ibrahim Khadra 1 , Clive G. Wilson 1 , Gavin W. Halbert 1
Affiliation
|
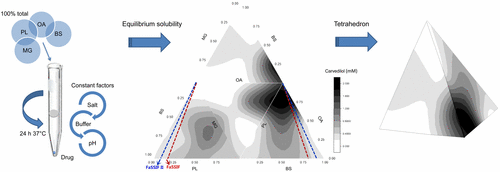
The absorption of poorly water-soluble drugs is influenced by the luminal gastrointestinal fluid content and composition, which control solubility. Simulated intestinal fluids have been introduced into dissolution testing including endogenous amphiphiles and digested lipids at physiological levels; however, in vivo individual variation exists in the concentrations of these components, which will alter drug absorption through an effect on solubility. The use of a factorial design of experiment and varying media by introducing different levels of bile, lecithin, and digested lipids has been previously reported, but here we investigate the solubility variation of poorly soluble drugs through more complex biorelevant amphiphile interactions. A four-component mixture design was conducted to understand the solubilization capacity and interactions of bile salt, lecithin, oleate, and monoglyceride with a constant total concentration (11.7 mM) but varying molar ratios. The equilibrium solubility of seven low solubility acidic (zafirlukast), basic (aprepitant, carvedilol), and neutral (fenofibrate, felodipine, griseofulvin, and spironolactone) drugs was investigated. Solubility results are comparable with literature values and also our own previously published design of experiment studies. Results indicate that solubilization is not a sum accumulation of individual amphiphile concentrations, but a drug specific effect through interactions of mixed amphiphile compositions with the drug. This is probably due to a combined interaction of drug characteristics; for example, lipophilicity, molecular shape, and ionization with amphiphile components, which can generate specific drug–micelle affinities. The proportion of each component can have a remarkable influence on solubility with, in some cases, the highest and lowest points close to each other. A single-point solubility measurement in a fixed composition simulated media or human intestinal fluid sample will therefore provide a value without knowledge of the surrounding solubility topography meaning that variability may be overlooked. This study has demonstrated how the amphiphile ratios influence drug solubility and highlights the importance of the envelope of physiological variation when simulating in vivo drug behavior.
中文翻译:

胃肠道表面活性剂比率对四组分混合设计研究的BCS II类药物平衡溶解度的影响
水溶性差的药物的吸收受腔内胃肠道液体含量和组成的影响,该含量和组成控制溶解性。已将模拟肠液引入溶出度测试,包括内源性两亲物和生理水平的消化脂质。但是,体内这些成分的浓度存在个体差异,这将通过影响溶解度来改变药物吸收。先前已经报道了通过引入不同水平的胆汁,卵磷脂和消化的脂质进行的因子设计实验和各种培养基的研究,但是在这里,我们通过与生物相关的两亲分子之间更复杂的相互作用来研究难溶性药物的溶解度变化。进行了四组分混合物设计,以了解具有恒定总浓度(11.7 mM)但摩尔比变化的胆盐,卵磷脂,油酸酯和甘油单酸酯的增溶能力和相互作用。研究了7种低溶解度的酸性(扎鲁司特),碱性(阿瑞匹坦,卡维地洛)和中性(非诺贝特,非洛地平,灰黄霉素和螺内酯)药物的平衡溶解度。溶解度结果可与文献数据相媲美,也可与我们自己先前发表的实验研究设计相媲美。结果表明增溶不是各个两亲物浓度的总和,而是通过混合两亲物组合物与药物相互作用的药物特异性作用。这可能是由于药物特性的相互作用所致。例如,亲脂性,分子形状以及两亲性成分的电离作用,会产生特定的药物-胶束亲和力。每种成分的比例可能会对溶解度产生显着影响,在某些情况下,最高点和最低点彼此接近。因此,在固定成分的模拟介质或人肠液样本中的单点溶解度测量将提供一个值,而无需了解周围的溶解度形貌,这意味着可能会忽略变异性。这项研究证明了两亲物比率如何影响药物溶解度,并强调了模拟时生理变化范围的重要性。体内药物行为。
更新日期:2017-08-22
中文翻译:

胃肠道表面活性剂比率对四组分混合设计研究的BCS II类药物平衡溶解度的影响
水溶性差的药物的吸收受腔内胃肠道液体含量和组成的影响,该含量和组成控制溶解性。已将模拟肠液引入溶出度测试,包括内源性两亲物和生理水平的消化脂质。但是,体内这些成分的浓度存在个体差异,这将通过影响溶解度来改变药物吸收。先前已经报道了通过引入不同水平的胆汁,卵磷脂和消化的脂质进行的因子设计实验和各种培养基的研究,但是在这里,我们通过与生物相关的两亲分子之间更复杂的相互作用来研究难溶性药物的溶解度变化。进行了四组分混合物设计,以了解具有恒定总浓度(11.7 mM)但摩尔比变化的胆盐,卵磷脂,油酸酯和甘油单酸酯的增溶能力和相互作用。研究了7种低溶解度的酸性(扎鲁司特),碱性(阿瑞匹坦,卡维地洛)和中性(非诺贝特,非洛地平,灰黄霉素和螺内酯)药物的平衡溶解度。溶解度结果可与文献数据相媲美,也可与我们自己先前发表的实验研究设计相媲美。结果表明增溶不是各个两亲物浓度的总和,而是通过混合两亲物组合物与药物相互作用的药物特异性作用。这可能是由于药物特性的相互作用所致。例如,亲脂性,分子形状以及两亲性成分的电离作用,会产生特定的药物-胶束亲和力。每种成分的比例可能会对溶解度产生显着影响,在某些情况下,最高点和最低点彼此接近。因此,在固定成分的模拟介质或人肠液样本中的单点溶解度测量将提供一个值,而无需了解周围的溶解度形貌,这意味着可能会忽略变异性。这项研究证明了两亲物比率如何影响药物溶解度,并强调了模拟时生理变化范围的重要性。体内药物行为。











































 京公网安备 11010802027423号
京公网安备 11010802027423号