当前位置:
X-MOL 学术
›
Mol. Biosyst.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Peculiarities of thermal denaturation of OmpF porin from Yersinia ruckeri
Molecular BioSystems Pub Date : 2017-07-20 00:00:00 , DOI: 10.1039/c7mb00239d Olga D. Novikova 1, 2, 3, 4, 5 , Dmitry K. Chistyulin 1, 2, 3, 4, 5 , Valentina A. Khomenko 1, 2, 3, 4, 5 , Evgeny V. Sidorin 1, 2, 3, 4, 5 , Natalya Yu. Kim 1, 2, 3, 4, 5 , Nina M. Sanina 4, 5, 6 , Olga Yu. Portnyagina 1, 2, 3, 4, 5 , Tamara F. Solov'eva 1, 2, 3, 4, 5 , Vladimir N. Uversky 7, 8, 9, 10, 11 , Valery L. Shnyrov 12, 13, 14, 15
Molecular BioSystems Pub Date : 2017-07-20 00:00:00 , DOI: 10.1039/c7mb00239d Olga D. Novikova 1, 2, 3, 4, 5 , Dmitry K. Chistyulin 1, 2, 3, 4, 5 , Valentina A. Khomenko 1, 2, 3, 4, 5 , Evgeny V. Sidorin 1, 2, 3, 4, 5 , Natalya Yu. Kim 1, 2, 3, 4, 5 , Nina M. Sanina 4, 5, 6 , Olga Yu. Portnyagina 1, 2, 3, 4, 5 , Tamara F. Solov'eva 1, 2, 3, 4, 5 , Vladimir N. Uversky 7, 8, 9, 10, 11 , Valery L. Shnyrov 12, 13, 14, 15
Affiliation
|
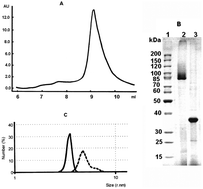
Irreversible denaturation of membrane proteins in detergent solutions is similar to unfolding of water-soluble multidomain proteins and represents a complex, multistage process. Pore-forming proteins of Gram-negative bacteria are heat-modifiable proteins, i.e., proteins altering their molecular forms (trimers or monomers), and accordingly, their electrophoretic mobilities depending upon denaturation conditions. There are still some contradictory data on the peculiarities of the conformational changes in the porin structure with temperature. Some authors demonstrated the loss of the porin trimeric structure only after unfolding of monomer subunits. Other researchers initially observed the dissociation of porin oligomers into the folded monomers. Using SDS-PAGE, spectroscopic methods and differential scanning calorimetry, a detailed study of thermally induced changes in the spatial structure of OmpF porin from the fish pathogen Yersinia ruckeri (Yr-OmpF) was carried out. The data obtained allowed us to conclude unambiguously that changes in the spatial structure of the monomers of Yr-OmpF precede the dissociation of the porin trimer.
中文翻译:

产耶尔森菌的OmpF孔蛋白热变性的特殊性
清洁剂溶液中膜蛋白的不可逆变性类似于水溶性多结构域蛋白的展开,代表了一个复杂的,多阶段的过程。革兰氏阴性细菌的毛孔形成蛋白是可热修饰的蛋白,即,蛋白质会改变其分子形式(三聚体或单体),并因此改变其电泳迁移率,具体取决于变性条件。关于孔蛋白结构随温度的构象变化的特殊性,仍然存在一些矛盾的数据。一些作者证明只有在单体亚基解折叠后,孔蛋白三聚体结构才会丢失。其他研究人员最初观察到孔蛋白低聚物解离为折叠的单体。使用SDS-PAGE,光谱法和差示扫描量热法,详细研究了热诱导鱼类致病性耶尔森氏菌OmpF孔蛋白的空间结构变化(Yr-OmpF)进行。获得的数据使我们能够明确地得出结论,Yr-OmpF单体的空间结构发生变化,这是孔三聚体解离之前的结果。
更新日期:2017-08-22
中文翻译:

产耶尔森菌的OmpF孔蛋白热变性的特殊性
清洁剂溶液中膜蛋白的不可逆变性类似于水溶性多结构域蛋白的展开,代表了一个复杂的,多阶段的过程。革兰氏阴性细菌的毛孔形成蛋白是可热修饰的蛋白,即,蛋白质会改变其分子形式(三聚体或单体),并因此改变其电泳迁移率,具体取决于变性条件。关于孔蛋白结构随温度的构象变化的特殊性,仍然存在一些矛盾的数据。一些作者证明只有在单体亚基解折叠后,孔蛋白三聚体结构才会丢失。其他研究人员最初观察到孔蛋白低聚物解离为折叠的单体。使用SDS-PAGE,光谱法和差示扫描量热法,详细研究了热诱导鱼类致病性耶尔森氏菌OmpF孔蛋白的空间结构变化(Yr-OmpF)进行。获得的数据使我们能够明确地得出结论,Yr-OmpF单体的空间结构发生变化,这是孔三聚体解离之前的结果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号