当前位置:
X-MOL 学术
›
Mol. Biosyst.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The antiproliferative activity of di-2-pyridylketone dithiocarbamate is partly attributed to catalase inhibition: detailing the interaction by spectroscopic methods
Molecular BioSystems Pub Date : 2017-06-30 00:00:00 , DOI: 10.1039/c7mb00032d Cuiping Li 1, 2, 3, 4 , Youxun Liu 1, 2, 3, 4 , Yun Fu 1, 2, 3, 4 , Tengfei Huang 1, 2, 3, 4, 5 , Lixia Kang 3, 4, 6, 7 , Changzheng Li 1, 2, 3, 4, 5
Molecular BioSystems Pub Date : 2017-06-30 00:00:00 , DOI: 10.1039/c7mb00032d Cuiping Li 1, 2, 3, 4 , Youxun Liu 1, 2, 3, 4 , Yun Fu 1, 2, 3, 4 , Tengfei Huang 1, 2, 3, 4, 5 , Lixia Kang 3, 4, 6, 7 , Changzheng Li 1, 2, 3, 4, 5
Affiliation
|
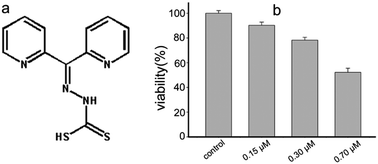
The bioactivity of drugs is attributed to their interaction with biological molecules, embodied in either their direct or indirect influence on enzyme activity and conformation. Di-2-pyridylketone hydrazine dithiocarbamate (DpdtC) exhibits significant antitumor activity in our preliminary study. We speculated that its activity may partly stem from enzyme inhibition due to strong metal chelating ability. To this end, we assessed its effect on catalase from erythrocytes and found evidence of inhibition, which was further confirmed by ROS determination in vivo. Thus, detailing the interaction between the agent and catalase via spectroscopic methods and molecular docking was required to obtain information on both the dynamics and thermodynamic parameters. The Lineweaver–Burk plot implied an uncompetitive pattern between DpdtC and catalase from beef liver, and IC50 = ∼7 μM. The thermodynamic parameters from fluorescence quenching measurements indicated that DpdtC could bind to catalase with moderate affinity (Ka = approximately 104 M−1). CD spectra revealed that DpdtC could significantly disrupt the secondary structure of catalase. Docking studies indicated that DpdtC bound to a flexible region of catalase, involving hydrogen bonds and salt bond; this was consistent with thermodynamic results from spectral investigations. Our data clearly showed that catalase inhibition of DpdtC was not due to direct chelation of iron from heme (killing), but through an allosteric effect. Thus, it can be concluded that the antiproliferative activity of DpdtC is partially attributed to its catalase inhibition.
中文翻译:

二-2-吡啶基酮二硫代氨基甲酸酯的抗增殖活性部分归因于过氧化氢酶的抑制作用:通过光谱方法详细说明了相互作用
药物的生物活性归因于它们与生物分子的相互作用,体现为它们对酶活性和构象的直接或间接影响。在我们的初步研究中,二-2-吡啶基肼肼二硫代氨基甲酸酯(DpdtC)表现出显着的抗肿瘤活性。我们推测其活性可能部分归因于强大的金属螯合能力而对酶的抑制作用。为此,我们评估了其对红细胞对过氧化氢酶的作用,并发现了抑制作用的证据,这在体内通过ROS测定得到了进一步证实。因此,通过需要光谱方法和分子对接来获得有关动力学和热力学参数的信息。Lineweaver-Burk图表明DpdtC和牛肝中的过氧化氢酶之间没有竞争模式,IC 50 =约7μM。荧光猝灭测量的热力学参数表明DpdtC可以中等亲和力与过氧化氢酶结合(K a =约10 4 M -1)。CD光谱表明,DpdtC可以显着破坏过氧化氢酶的二级结构。对接研究表明,DpdtC结合过氧化氢酶的柔性区域,涉及氢键和盐键。这与光谱研究的热力学结果一致。我们的数据清楚地表明,DpdtC的过氧化氢酶抑制作用不是由于血红素对铁的直接螯合(杀死),而是由于变构作用。因此,可以得出结论,DpdtC的抗增殖活性部分归因于其过氧化氢酶抑制作用。
更新日期:2017-08-22
中文翻译:

二-2-吡啶基酮二硫代氨基甲酸酯的抗增殖活性部分归因于过氧化氢酶的抑制作用:通过光谱方法详细说明了相互作用
药物的生物活性归因于它们与生物分子的相互作用,体现为它们对酶活性和构象的直接或间接影响。在我们的初步研究中,二-2-吡啶基肼肼二硫代氨基甲酸酯(DpdtC)表现出显着的抗肿瘤活性。我们推测其活性可能部分归因于强大的金属螯合能力而对酶的抑制作用。为此,我们评估了其对红细胞对过氧化氢酶的作用,并发现了抑制作用的证据,这在体内通过ROS测定得到了进一步证实。因此,通过需要光谱方法和分子对接来获得有关动力学和热力学参数的信息。Lineweaver-Burk图表明DpdtC和牛肝中的过氧化氢酶之间没有竞争模式,IC 50 =约7μM。荧光猝灭测量的热力学参数表明DpdtC可以中等亲和力与过氧化氢酶结合(K a =约10 4 M -1)。CD光谱表明,DpdtC可以显着破坏过氧化氢酶的二级结构。对接研究表明,DpdtC结合过氧化氢酶的柔性区域,涉及氢键和盐键。这与光谱研究的热力学结果一致。我们的数据清楚地表明,DpdtC的过氧化氢酶抑制作用不是由于血红素对铁的直接螯合(杀死),而是由于变构作用。因此,可以得出结论,DpdtC的抗增殖活性部分归因于其过氧化氢酶抑制作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号