Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2017-08-22 , DOI: 10.1016/j.bmc.2017.08.036 Ismail M. Taban , Jinge Zhu , Hector F. DeLuca , Claire Simons
|
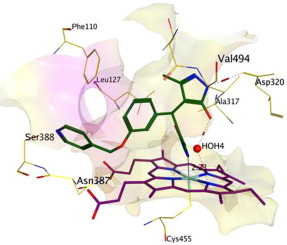
A homology model of human CYP27B1 was built using MOE and was further optimised by molecular dynamics simulations of the hCYP27B1 homology model and a hCYP27B1-SDZ-88357 complex. Docking results from the hCYP27B1-SDZ-88357 complex showed amino acids Arg107, Asn387 and Asp320 have an important role in binding interaction, with Asp320 part of the important acid-alcohol pair situated in the I-helix with the conserved sequence (A/G) GX (E/D) (T/S), which assumes an essential role in the binding of an oxygen molecule for catalysis. Additional docking experiments with selective hCYP27B1 or hCYP24A1 inhibitors using both the hCYP27B1 model and a triple mutant hCYP24A1 model provided further support for the importance of H-bonding interactions with the three identified active site amino acids. To confirm the role of Arg107, Asn387 and Asp320 in the active site of hCYP27B1 compounds were designed that would form H-bonding interactions, as determined from docking experiments with the hCYP27B1 model. Subsequent synthesis and CYP24A1 and CYP27B1 enzyme assays of the designed compounds 1a and 1b showed a ∼5-fold selectivity for CYP27B1 confirming the importance of Asp320 in particular and also Asn387 and Arg107 as important amino acids for CYP27B1 inhibitory activity.
中文翻译:

分析维生素D1α-羟化酶(CYP27B1)和维生素D 24-羟化酶(CYP24A1)的结合位点以设计选择性CYP24A1抑制剂:同源性建模,分子动力学模拟和关键结合要求的鉴定
使用MOE建立了人CYP27B1的同源性模型,并通过hCYP27B1同源性模型和hCYP27B1-SDZ-88357复合物的分子动力学模拟对其进行了进一步优化。hCYP27B1-SDZ-88357复合物的对接结果表明,氨基酸Arg107,Asn387和Asp320在结合相互作用中具有重要作用,其中重要的酸-醇对的Asp320部分位于I-螺旋中,具有保守的序列(A / G )GX(E / D)(T / S),在催化氧分子的结合中起着至关重要的作用。使用hCYP27B1模型和三突变体hCYP24A1模型与选择性hCYP27B1或hCYP24A1抑制剂进行的其他对接实验,为与三个已鉴定的活性位点氨基酸进行H键相互作用的重要性提供了进一步的支持。为了确认Arg107的作用,根据与hCYP27B1模型的对接实验确定,设计的hCYP27B1化合物活性位点中的Asn387和Asp320会形成H键相互作用。后续合成以及所设计化合物的CYP24A1和CYP27B1酶测定图1a和1b显示出对CYP27B1的约5倍的选择性,这证实了Asp320的重要性,特别是确认了Asn387和Arg107作为CYP27B1抑制活性的重要氨基酸。











































 京公网安备 11010802027423号
京公网安备 11010802027423号