Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Health-related quality of life for everolimus versus placebo in patients with advanced, non-functional, well-differentiated gastrointestinal or lung neuroendocrine tumours (RADIANT-4): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial.
The Lancet ( IF 98.4 ) Pub Date : 2017-08-30 , DOI: 10.1016/s1470-2045(17)30471-0 Marianne E Pavel 1 , Simron Singh 2 , Jonathan R Strosberg 3 , Lida Bubuteishvili-Pacaud 4 , Evgeny Degtyarev 4 , Maureen P Neary 5 , Carlo Carnaghi 6 , Jiri Tomasek 7 , Edward Wolin 8 , Markus Raderer 9 , Harald Lahner 10 , Juan W Valle 11 , Rodney Pommier 12 , Eric Van Cutsem 13 , Margot E T Tesselaar 14 , Gianfranco Delle Fave 15 , Roberto Buzzoni 16 , Matthias Hunger 17 , Jennifer Eriksson 18 , David Cella 19 , Jean-François Ricci 20 , Nicola Fazio 21 , Matthew H Kulke 22 , James C Yao 23
The Lancet ( IF 98.4 ) Pub Date : 2017-08-30 , DOI: 10.1016/s1470-2045(17)30471-0 Marianne E Pavel 1 , Simron Singh 2 , Jonathan R Strosberg 3 , Lida Bubuteishvili-Pacaud 4 , Evgeny Degtyarev 4 , Maureen P Neary 5 , Carlo Carnaghi 6 , Jiri Tomasek 7 , Edward Wolin 8 , Markus Raderer 9 , Harald Lahner 10 , Juan W Valle 11 , Rodney Pommier 12 , Eric Van Cutsem 13 , Margot E T Tesselaar 14 , Gianfranco Delle Fave 15 , Roberto Buzzoni 16 , Matthias Hunger 17 , Jennifer Eriksson 18 , David Cella 19 , Jean-François Ricci 20 , Nicola Fazio 21 , Matthew H Kulke 22 , James C Yao 23
Affiliation
|
BACKGROUND
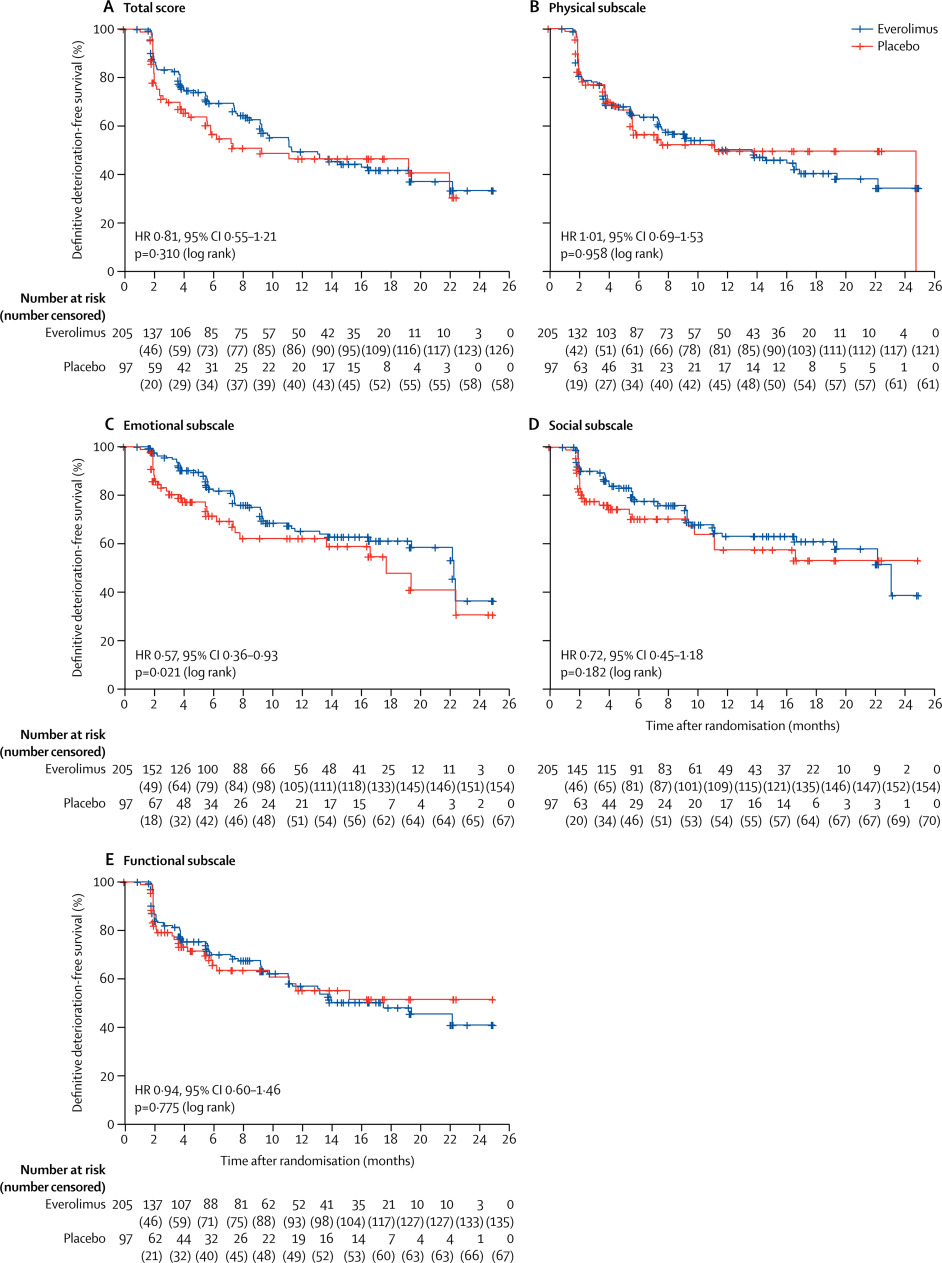
In the phase 3 RADIANT-4 trial, everolimus increased progression-free survival compared with placebo in patients with advanced, progressive, non-functional, well-differentiated gastrointestinal or lung neuroendocrine tumours (NETs). We now report the health-related quality of life (HRQOL) secondary endpoint.
METHODS
RADIANT-4 is a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial done in 97 centres in 25 countries worldwide. Adults (aged ≥18 years) were eligible for the study if they had pathologically confirmed, advanced (unresectable or metastatic), non-functional, well-differentiated (grade 1 or 2) NETs of lung or gastrointestinal origin. Patients were randomly allocated (2:1) using block randomisation (block size of three) by an interactive voice response system to receive oral everolimus (10 mg per day) or placebo, both with best supportive care, with stratification by tumour origin, WHO performance status, and previous somatostatin analogue treatment. HRQOL was assessed with the Functional Assessment of Cancer Therapy-General (FACT-G) questionnaire at baseline (visit 2, day 1), every 8 weeks (± 1 week) during the study for the first 12 months after randomisation, and every 12 weeks thereafter until study drug discontinuation. The primary endpoint, reported previously, was progression-free survival assessed by central review; HRQOL was a prespecified secondary endpoint. The prespecified secondary outcome measure was time to definitive deterioration (≥7 points) in FACT-G total score. Analyses were done on the full analysis set, consisting of all randomised patients, by intention to treat. Only data obtained while receiving the randomly allocated treatment were included in this analysis. Enrolment for RADIANT-4 was completed on Aug 23, 2013, but the trial is ongoing pending final analysis of the key secondary endpoint of overall survival. This trial is registered with ClinicalTrials.gov, number NCT01524783.
FINDINGS
Between April 3, 2012, and Aug 23, 2013, 302 patients were enrolled; 205 were randomly allocated everolimus and 97 were assigned placebo. At baseline, 193 (94%) of 205 patients assigned everolimus and 95 (98%) of 97 allocated placebo had completed either fully or partly the FACT-G questionnaire; at week 48, 70 (83%) of 84 patients assigned everolimus and 22 (85%) of 26 allocated placebo completed FACT-G. Median time to definitive deterioration in FACT-G total score was 11·27 months (95% CI 9·27-19·35) with everolimus and 9·23 months (5·52-not estimable) with placebo (adjusted hazard ratio 0·81, 95% CI 0·55-1·21; log-rank p=0·31).
INTERPRETATION
HRQOL was maintained for patients with advanced, non-functional, gastrointestinal or lung NETs, with no relevant differences noted between the everolimus and placebo groups. In view of the previous RADIANT-4 findings of longer progression-free survival with everolimus, our findings suggest that everolimus delays disease progression while preserving overall HRQOL, even with the usual toxic effects related to active targeted drug treatment for cancer.
FUNDING
Novartis Pharmaceuticals.
中文翻译:

患有晚期,无功能,高度分化的胃肠道或肺神经内分泌肿瘤(RADIANT-4)的患者,依维莫司和安慰剂的健康相关生活质量:一项多中心,随机,双盲,安慰剂对照的3期试验。
背景技术在3期RADIANT-4试验中,与安慰剂相比,依维莫司在患有晚期,进行性,非功能性,高度分化的胃肠道或肺神经内分泌肿瘤(NETs)的患者中提高了无进展生存期。现在,我们报告与健康有关的生活质量(HRQOL)次要终点。方法RADIANT-4是一项在全球25个国家/地区的97个中心进行的多中心,随机,双盲,安慰剂对照的3期临床试验。如果成年人(≥18岁)具有经病理学证实,晚期(不可切除或转移性),无功能,分化良好(1级或2级)的肺或胃肠道NET,则符合研究条件。患者被随机分配(2:1)通过互动式语音应答系统使用区组随机化(区组大小为三个)接受口服依维莫司(每天10 mg)或安慰剂,均获得最佳支持治疗,并按肿瘤起源,WHO表现状态和以前的生长抑素类似物分层治疗。HRQOL在基线后(访问第2天,第1天),研究后每8周(±1周),随机分组后的前12个月,以及每12个月,均用癌症一般治疗功能评估(FACT-G)问卷进行了评估。此后数周,直到研究药物停药。先前报道的主要终点是通过中心评估评估的无进展生存期。HRQOL是预先指定的次要终点。预先指定的次要结局指标是FACT-G总评分达到确定的恶化时间(≥7分)的时间。根据意向治疗,对由所有随机分组患者组成的完整分析集进行了分析。该分析仅包括接受随机分配治疗时获得的数据。RADIANT-4的注册已于2013年8月23日完成,但该试验仍在进行中,尚待对总体生存的关键次要终点进行最终分析。该试验已在ClinicalTrials.gov上注册,编号为NCT01524783。结果在2012年4月3日至2013年8月23日之间,共招募302例患者。随机分配了205个依维莫司,分配了97个安慰剂。在基线时,分配了依维莫司的205名患者中的193名(94%)和分配了97名安慰剂的95名(98%)已全部或部分完成FACT-G调查问卷;在第48周,分配了依维莫司的84名患者中有70名(83%)和分配的26名安慰剂中的22名(85%)完成了FACT-G。依维莫司FACT-G总体评分最终恶化的中位时间为11·27个月(95%CI 9·27-19·35),安慰剂为9·23个月(5·52-不可估计)(危险比调整为0) ·81,95%CI 0·55-1·21;对数秩p = 0·31)。解释HRQOL适用于晚期,无功能,胃肠道或肺部NET的患者,依维莫司组与安慰剂组之间无相关差异。鉴于以前的RADIANT-4研究结果显示,依维莫司具有更长的无进展生存期,我们的发现表明依维莫司可延缓疾病进展,同时保留总体HRQOL,甚至具有与癌症主动靶向药物治疗相关的常见毒性作用。资金诺华制药。
更新日期:2017-08-22
中文翻译:

患有晚期,无功能,高度分化的胃肠道或肺神经内分泌肿瘤(RADIANT-4)的患者,依维莫司和安慰剂的健康相关生活质量:一项多中心,随机,双盲,安慰剂对照的3期试验。
背景技术在3期RADIANT-4试验中,与安慰剂相比,依维莫司在患有晚期,进行性,非功能性,高度分化的胃肠道或肺神经内分泌肿瘤(NETs)的患者中提高了无进展生存期。现在,我们报告与健康有关的生活质量(HRQOL)次要终点。方法RADIANT-4是一项在全球25个国家/地区的97个中心进行的多中心,随机,双盲,安慰剂对照的3期临床试验。如果成年人(≥18岁)具有经病理学证实,晚期(不可切除或转移性),无功能,分化良好(1级或2级)的肺或胃肠道NET,则符合研究条件。患者被随机分配(2:1)通过互动式语音应答系统使用区组随机化(区组大小为三个)接受口服依维莫司(每天10 mg)或安慰剂,均获得最佳支持治疗,并按肿瘤起源,WHO表现状态和以前的生长抑素类似物分层治疗。HRQOL在基线后(访问第2天,第1天),研究后每8周(±1周),随机分组后的前12个月,以及每12个月,均用癌症一般治疗功能评估(FACT-G)问卷进行了评估。此后数周,直到研究药物停药。先前报道的主要终点是通过中心评估评估的无进展生存期。HRQOL是预先指定的次要终点。预先指定的次要结局指标是FACT-G总评分达到确定的恶化时间(≥7分)的时间。根据意向治疗,对由所有随机分组患者组成的完整分析集进行了分析。该分析仅包括接受随机分配治疗时获得的数据。RADIANT-4的注册已于2013年8月23日完成,但该试验仍在进行中,尚待对总体生存的关键次要终点进行最终分析。该试验已在ClinicalTrials.gov上注册,编号为NCT01524783。结果在2012年4月3日至2013年8月23日之间,共招募302例患者。随机分配了205个依维莫司,分配了97个安慰剂。在基线时,分配了依维莫司的205名患者中的193名(94%)和分配了97名安慰剂的95名(98%)已全部或部分完成FACT-G调查问卷;在第48周,分配了依维莫司的84名患者中有70名(83%)和分配的26名安慰剂中的22名(85%)完成了FACT-G。依维莫司FACT-G总体评分最终恶化的中位时间为11·27个月(95%CI 9·27-19·35),安慰剂为9·23个月(5·52-不可估计)(危险比调整为0) ·81,95%CI 0·55-1·21;对数秩p = 0·31)。解释HRQOL适用于晚期,无功能,胃肠道或肺部NET的患者,依维莫司组与安慰剂组之间无相关差异。鉴于以前的RADIANT-4研究结果显示,依维莫司具有更长的无进展生存期,我们的发现表明依维莫司可延缓疾病进展,同时保留总体HRQOL,甚至具有与癌症主动靶向药物治疗相关的常见毒性作用。资金诺华制药。











































 京公网安备 11010802027423号
京公网安备 11010802027423号