当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gram-Scale, Stereoselective Synthesis and Biological Evaluation of (+)-Armillariol C
Journal of Natural Products ( IF 3.3 ) Pub Date : 2017-08-21 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00484 M. Damoder Reddy 1 , Hajime Kobori , Takumi Mori , Jing Wu , Hirokazu Kawagishi , E. Blake Watkins 1
Journal of Natural Products ( IF 3.3 ) Pub Date : 2017-08-21 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00484 M. Damoder Reddy 1 , Hajime Kobori , Takumi Mori , Jing Wu , Hirokazu Kawagishi , E. Blake Watkins 1
Affiliation
|
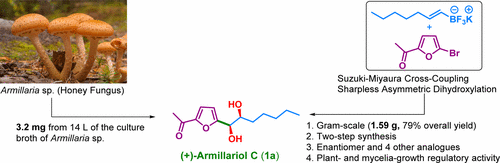
Natural products with heteroaromatic cores are ample and widespread in nature, with many compounds exhibiting promising therapeutic properties. (+)-Armillariol C (1a) is a furan-based natural product isolated from Armillaria species. Herein, we report the first enantioselective synthesis of (+)-armillariol C (1a, 79% overall yield), its enantiomer (1b), and four other analogues, on a gram-scale, using microwave-mediated, Suzuki–Miyaura cross-coupling and Sharpless asymmetric dihydroxylation reactions. Compounds were tested for plant- and mycelia-growth regulatory activity, with 1b, 7a, and 7b showing the strongest inhibitory properties in a lettuce assay and 7b and 9b inhibiting Flammulina velutipes.
中文翻译:

(+)-Armillariol C的克级,立体选择性合成和生物学评估
具有杂芳族核心的天然产物在自然界中是丰富而广泛的,许多化合物表现出有希望的治疗特性。(+)- Armillariol C(1a)是一种从呋喃菌属物种中分离出来的基于呋喃的天然产物。在此,我们报道了使用微波介导的Suzuki-Miyaura杂交技术以克为单位的(+)-蜜环丙醇C(1a,总收率79%),其对映异构体(1b)和其他四个类似物的首次对映选择性合成。偶联和Sharpless不对称二羟基化反应。测试了化合物对植物和菌丝体生长的调节活性,分别为1b,7a和7b在生菜试验中表现出最强的抑制作用,并抑制7b和9b的金针菇。
更新日期:2017-08-21
中文翻译:

(+)-Armillariol C的克级,立体选择性合成和生物学评估
具有杂芳族核心的天然产物在自然界中是丰富而广泛的,许多化合物表现出有希望的治疗特性。(+)- Armillariol C(1a)是一种从呋喃菌属物种中分离出来的基于呋喃的天然产物。在此,我们报道了使用微波介导的Suzuki-Miyaura杂交技术以克为单位的(+)-蜜环丙醇C(1a,总收率79%),其对映异构体(1b)和其他四个类似物的首次对映选择性合成。偶联和Sharpless不对称二羟基化反应。测试了化合物对植物和菌丝体生长的调节活性,分别为1b,7a和7b在生菜试验中表现出最强的抑制作用,并抑制7b和9b的金针菇。











































 京公网安备 11010802027423号
京公网安备 11010802027423号