当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
From Drying Kinetics, Solvate Structure, Particle Morphology, and Modeling to Optimal Drying Protocol
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2017-08-21 00:00:00 , DOI: 10.1021/acs.oprd.7b00162 Daniel S. Hsieh 1 , Qi Gao 1 , Ming Huang 1 , Lynn M. DiMemmo 1 , Mark Lindrud 1 , Thomas Razler 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2017-08-21 00:00:00 , DOI: 10.1021/acs.oprd.7b00162 Daniel S. Hsieh 1 , Qi Gao 1 , Ming Huang 1 , Lynn M. DiMemmo 1 , Mark Lindrud 1 , Thomas Razler 1
Affiliation
|
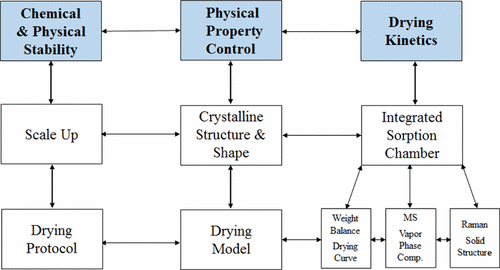
The mechanistic understanding of three key active pharmaceutical ingredient (API) drying elements: drying kinetics, physical property control, and stability control, is critical for downstream formulation development and manufacture. The objective of this study was to collect drying kinetic data through the use of a laboratory integrated sorption chamber to elucidate the drying mechanism and thereby establish a drying model. In addition, the collection of drying kinetic data would provide a greater understanding of the solvent to API ratio as well as the API stability to help develop a large-scale drying protocol. The study material is an ethanol solvate (ethanolate) of the API. There is no fundamental crystal structure change upon drying of the solvate in the temperature range from 50 to 90 °C, and the drying kinetic data show that the molar ratio of ethanol to API is 1:1. This is confirmed by X-ray crystallography. An analysis of the drying kinetics showed that the diffusion of ethanol through the crystal lattice provided the primary mass transfer resistance, which can be simulated using the Fickian principle of diffusion through a slab. The diffusion coefficients from 50 to 90 °C at 20 mmHg were found to be strongly temperature-dependent and fit well using an Arrhenius type relationship. The activation energy for diffusion was then determined from this relationship. By using these diffusion parameters, the drying curves at various temperatures were generated to predict the drying time to meet the final ethanol specification. The predicted drying time was in good agreement with experimental drying data at high temperatures (80 and 90 °C) but shorter than that observed when drying at lower temperatures (between 50 and 70 °C). Thermal stability data showed that the compound is stable at drying temperatures equal or below 70 °C. Drying data from this study are generated from a static balance. Hence, the effect of shear forces imposed from cake depth and dryer agitation on particle morphology, physical stability of the desolvated API, and drying time needs to be studied to generate the optimal drying protocol for large-scale operation.
中文翻译:

从干燥动力学,溶剂化物结构,颗粒形态和建模到最佳干燥方案
对三个关键活性药物成分(API)干燥元素的机械理解:干燥动力学,物理性能控制和稳定性控制,对于下游制剂的开发和制造至关重要。这项研究的目的是通过使用实验室集成的吸附室来收集干燥动力学数据,以阐明干燥机理,从而建立干燥模型。此外,干燥动力学数据的收集将提供对溶剂与API比率以及API稳定性的更好理解,以帮助开发大规模干燥方案。研究材料是API的乙醇溶剂化物(乙醇化物)。在50至90°C的温度范围内干燥溶剂化物后,基本晶体结构没有变化,干燥动力学数据表明,乙醇与API的摩尔比为1:1。这通过X射线晶体学证实。对干燥动力学的分析表明,乙醇通过晶格的扩散提供了主要的传质阻力,这可以使用通过板坯扩散的Fickian原理进行模拟。发现在20 mmHg下从50到90°C的扩散系数与温度密切相关,并且使用Arrhenius类型关系拟合得很好。然后根据该关系确定扩散的活化能。通过使用这些扩散参数,可生成各种温度下的干燥曲线,以预测干燥时间以达到最终的乙醇规格。预测的干燥时间与高温(80和90°C)下的实验干燥数据非常吻合,但比在较低温度(50和70°C之间)干燥时观察到的时间短。热稳定性数据表明,该化合物在等于或低于70°C的干燥温度下稳定。这项研究的干燥数据是从静态天平得出的。因此,需要研究滤饼深度和干燥机搅动施加的剪切力对颗粒形态,去溶剂化API的物理稳定性以及干燥时间的影响,以生成用于大规模操作的最佳干燥方案。这项研究的干燥数据是从静态天平得出的。因此,需要研究滤饼深度和干燥机搅动施加的剪切力对颗粒形态,去溶剂化API的物理稳定性以及干燥时间的影响,以生成用于大规模操作的最佳干燥方案。这项研究的干燥数据是从静态天平得出的。因此,需要研究滤饼深度和干燥机搅动施加的剪切力对颗粒形态,去溶剂化API的物理稳定性以及干燥时间的影响,以生成用于大规模操作的最佳干燥方案。
更新日期:2017-08-21
中文翻译:

从干燥动力学,溶剂化物结构,颗粒形态和建模到最佳干燥方案
对三个关键活性药物成分(API)干燥元素的机械理解:干燥动力学,物理性能控制和稳定性控制,对于下游制剂的开发和制造至关重要。这项研究的目的是通过使用实验室集成的吸附室来收集干燥动力学数据,以阐明干燥机理,从而建立干燥模型。此外,干燥动力学数据的收集将提供对溶剂与API比率以及API稳定性的更好理解,以帮助开发大规模干燥方案。研究材料是API的乙醇溶剂化物(乙醇化物)。在50至90°C的温度范围内干燥溶剂化物后,基本晶体结构没有变化,干燥动力学数据表明,乙醇与API的摩尔比为1:1。这通过X射线晶体学证实。对干燥动力学的分析表明,乙醇通过晶格的扩散提供了主要的传质阻力,这可以使用通过板坯扩散的Fickian原理进行模拟。发现在20 mmHg下从50到90°C的扩散系数与温度密切相关,并且使用Arrhenius类型关系拟合得很好。然后根据该关系确定扩散的活化能。通过使用这些扩散参数,可生成各种温度下的干燥曲线,以预测干燥时间以达到最终的乙醇规格。预测的干燥时间与高温(80和90°C)下的实验干燥数据非常吻合,但比在较低温度(50和70°C之间)干燥时观察到的时间短。热稳定性数据表明,该化合物在等于或低于70°C的干燥温度下稳定。这项研究的干燥数据是从静态天平得出的。因此,需要研究滤饼深度和干燥机搅动施加的剪切力对颗粒形态,去溶剂化API的物理稳定性以及干燥时间的影响,以生成用于大规模操作的最佳干燥方案。这项研究的干燥数据是从静态天平得出的。因此,需要研究滤饼深度和干燥机搅动施加的剪切力对颗粒形态,去溶剂化API的物理稳定性以及干燥时间的影响,以生成用于大规模操作的最佳干燥方案。这项研究的干燥数据是从静态天平得出的。因此,需要研究滤饼深度和干燥机搅动施加的剪切力对颗粒形态,去溶剂化API的物理稳定性以及干燥时间的影响,以生成用于大规模操作的最佳干燥方案。







































 京公网安备 11010802027423号
京公网安备 11010802027423号