Nature Chemistry ( IF 19.2 ) Pub Date : 2017-08-21 , DOI: 10.1038/nchem.2846 Nicholas F. Polizzi , Yibing Wu , Thomas Lemmin , Alison M. Maxwell , Shao-Qing Zhang , Jeff Rawson , David N. Beratan , Michael J. Therien , William F. DeGrado
|
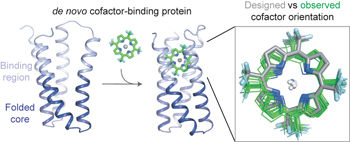
Protein catalysis requires the atomic-level orchestration of side chains, substrates and cofactors, and yet the ability to design a small-molecule-binding protein entirely from first principles with a precisely predetermined structure has not been demonstrated. Here we report the design of a novel protein, PS1, that binds a highly electron-deficient non-natural porphyrin at temperatures up to 100 °C. The high-resolution structure of holo-PS1 is in sub-Å agreement with the design. The structure of apo-PS1 retains the remote core packing of the holoprotein, with a flexible binding region that is predisposed to ligand binding with the desired geometry. Our results illustrate the unification of core packing and binding-site definition as a central principle of ligand-binding protein design.
中文翻译:

从头设计具有亚Å精度的超稳定非天然蛋白-配体复合物
蛋白质催化需要侧链,底物和辅因子的原子级协调,但是尚未证明完全根据第一原理设计出具有精确预定结构的小分子结合蛋白的能力。在这里,我们报告了一种新型蛋白质PS1的设计,该蛋白质在高达100°C的温度下结合高度电子缺陷的非天然卟啉。holo-PS1的高分辨率结构与设计相符。apo-PS1的结构保留了完整蛋白的远端核心堆积,并具有一个灵活的结合区域,该区域易于与具有所需几何形状的配体结合。我们的结果说明了核心包装和结合位点定义的统一是配体结合蛋白设计的主要原则。











































 京公网安备 11010802027423号
京公网安备 11010802027423号