Applied Catalysis A: General ( IF 4.7 ) Pub Date : 2017-08-19 , DOI: 10.1016/j.apcata.2017.08.021 Mawan Nugraha , Meng-Che Tsai , John Rick , Wei-Nien Su , Hung-Lung Chou , Bing Joe Hwang
|
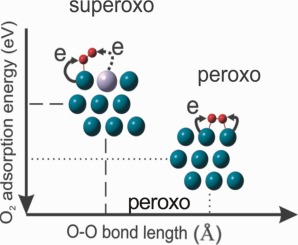
One of the main obstacles confronting the direct synthesis of hydrogen peroxide (H2O2) is how to maintain the unbroken OO bonding of the intermediate species on the catalytic surface. To address this challenge Pd-Hg alloys have been used with initial reports suggesting their performance offers advantages when compared to monometallic Pd and Pd-Au alloys; however, the interactions of O2 with Pd-Hg alloys are not well characterised. In this study, density functional theory (DFT) calculations, employed to investigate O2 adsorption on the Pd and Pd-Hg alloy surfaces, suggested O2 adsorption can occur via either a superoxo or a peroxo pathway and that when Hg is alloyed to Pd there are more surface adsorbed superoxo groups compared to adsorption on a monometallic Pd surface. The Hg in Pd6Hg3/Pd(111) results in an electronic surface structure different to that of Pd(111) and a reduced O2 adsorption energy. The stronger O2 surface interactions, when combined with weaker O
O bonding (of the adsorbed O2), which result from the presence of Hg on the Pd-Hg surface leads to synergistic geometric and electronic effects that result in an increased selectivity during of the synthesis of H2O2.
中文翻译:

DFT研究揭示了用于过氧化氢形成的钯汞合金催化剂的几何和电子协同作用
直接合成过氧化氢(H 2 O 2)面临的主要障碍之一是如何维持催化表面上中间物种的连续O O键。为了应对这一挑战,Pd-Hg合金已被用于初步报告,表明与单金属Pd和Pd-Au合金相比,其性能具有优势。然而,O 2与Pd-Hg合金的相互作用尚不能很好地表征。在这项研究中,用于研究O 2在Pd和Pd-Hg合金表面上的吸附的密度泛函理论(DFT)计算表明O 2吸附可通过超氧或过氧途径发生,并且当汞与Pd合金化时,与单金属Pd表面的吸附相比,表面吸附的超氧基团更多。Pd 6 Hg 3 / Pd(111)中的Hg导致电子表面结构与Pd(111)不同,并且O 2吸附能降低。当Pd-Hg表面上存在Hg时,与被吸附的O 2的较弱的O O键结合时,较强的O 2表面相互作用会导致协同的几何和电子效应,从而在分离过程中提高选择性。 H 2 O 2的合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号