Journal of Catalysis ( IF 6.5 ) Pub Date : 2017-08-19 , DOI: 10.1016/j.jcat.2017.07.033 Guangxing Yang , Lida M. Namin , N. Aaron Deskins , Xiaowei Teng
|
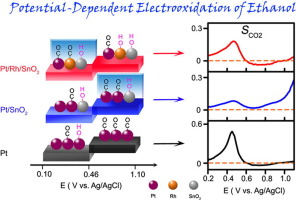
Direct ethanol fuel cells (DEFCs) are a promising technology for the generation of electricity via the direct conversion of ethanol into CO2, showing higher thermodynamic efficiency and volumetric energy density than hydrogen fuel cells. However, implementation of DEFCs is hampered by the low CO2 selectivity during the ethanol oxidation reaction (EOR). Comprehensive understanding of the electro-kinetics and reaction pathways of CO2 generation via CC bond-breaking is not only a fundamental question for electro-catalysis, but also a key technological challenge since practical implementation of DEFC technology is contingent on its ability to selectively oxidize ethanol into CO2 to achieve exceptional energy density through 12-electron transfer reaction. Here, we present comprehensive in situ potentiodynamics studies of CO2 generation during the EOR on Pt, Pt/SnO2 and Pt/Rh/SnO2 catalysts using a house-made electrochemical cell equipped with a CO2 microelectrode. Highly sensitive CO2 measurements enable the real time detection of the partial pressure of CO2 during linear sweep voltammetry measurements, through which electro-kinetics details of CO2 generation can be obtained. In situ CO2 measurements provide the mechanistic understanding of potentiodynamics of the EOR, particularly the influence of ∗OH adsorbates on CO2 generation rate and selectivity. Density functional theory (DFT) simulations of Pt, Pt/SnO2, and Pt/Rh/SnO2 surfaces clarify reaction details over these catalysts. Our results show that at low potentials, inadequate ∗OH adsorbates impair the removal of reaction intermediates, and thus Pt/Rh/SnO2 exhibited the best performance toward CO2 generation, while at high potentials, Rh sites were overwhelmingly occupied (poisoned) by ∗OH adsorbates, and thus Pt/SnO2 exhibited the best performance toward CO2 generation.
中文翻译:

* OH吸附物对乙醇电氧化过程中产生CO 2的电位动力学的影响
直接乙醇燃料电池(DEFC)是一种通过将乙醇直接转化为CO 2来发电的有前途的技术,与氢燃料电池相比,其显示出更高的热力学效率和体积能密度。但是,在乙醇氧化反应(EOR)期间,由于CO 2选择性低,阻碍了DEFC的实施。全面理解通过C C键断裂产生CO 2的动力学和反应途径不仅是电催化的基本问题,而且是关键的技术挑战,因为DEFC技术的实际实施取决于其选择性地选择碳的能力。将乙醇氧化成CO 2通过12电子转移反应实现出色的能量密度。在这里,我们介绍了使用配备有CO 2微电极的自制电化学电池在Pt,Pt / SnO 2和Pt / Rh / SnO 2催化剂上进行EOR期间CO 2生成的综合原位电动力学研究。高度敏感的CO 2测量值可以在线性扫描伏安法测量过程中实时检测CO 2的分压,从而可以获取产生CO 2的电动细节。原位CO 2测量结果提供了对EOR电位动力学的机械理解,尤其是* OH吸附物对CO 2生成速率和选择性的影响。Pt,Pt / SnO 2和Pt / Rh / SnO 2表面的密度泛函理论(DFT)模拟阐明了这些催化剂的反应细节。我们的结果表明,在低电势下,不足的* OH吸附物会阻碍反应中间体的去除,因此Pt / Rh / SnO 2表现出最佳的CO 2生成性能,而在高电势下,Rh位点被绝大多数占据(中毒)* OH吸附,因此Pt / SnO 2在产生CO 2方面表现出最好的性能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号