Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2017-08-19 , DOI: 10.1016/j.bmc.2017.08.029 Jinyu Yang , Gaoliang Cheng , Qihao Xu , Shenglin Luan , Shuxiang Wang , Dan Liu , Linxiang Zhao
|
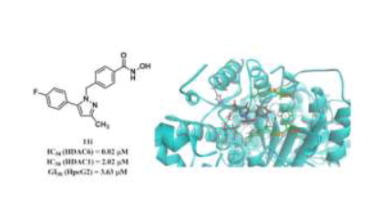
In recent years, inhibition of HDAC6 became a promising therapeutic strategy for the treatment of cancer and HDAC6 inhibitors were considered to be potent anti-cancer agents. In this work, celecoxib showed moderate degree of HDAC6 inhibition activity and selectivity in preliminary enzyme inhibition activity assay. A series of hydroxamic acid derivatives bearing phenylpyrazol moiety were designed and synthesized as HDAC6 inhibitors. Most compounds showed potent HDAC6 inhibition activity. 11i was the most selective compound against HDAC6 with IC50 values of 0.020 µM and selective factor of 101.1. Structure-activity relationship analysis indicated that locating the linker group at 1′ of pyrazol gave the most selectivity. The most compounds 11i (GI50 = 3.63 μM) exhibited 6-fold more potent than vorinostat in HepG2 cells. Considering of the high selectivity against HDAC6 and anti-proliferation activity, such compounds have potential to be developed as anti-cancer agents.
中文翻译:

以苯并吡唑骨架为表面识别基序的新型基于异羟肟酸的组蛋白脱乙酰基酶6选择性抑制剂的设计,合成和生物学评价
近年来,抑制HDAC6成为治疗癌症的有前途的治疗策略,并且HDAC6抑制剂被认为是有效的抗癌药。在这项工作中,塞来昔布在初步的酶抑制活性测定中显示出中等程度的HDAC6抑制活性和选择性。设计并合成了一系列带有苯并吡唑部分的异羟肟酸衍生物,作为HDAC6抑制剂。大多数化合物显示出有效的HDAC6抑制活性。11i是针对HDAC6的最具选择性的化合物,IC 50值为0.020 µM,选择性系数为101.1。结构-活性关系分析表明,在吡唑的1'端定位连接基团具有最大的选择性。最多的化合物11i(GI 50 = 3.63μM)在HepG2细胞中表现出的效力比伏立诺他高6倍。考虑到对HDAC6的高选择性和抗增殖活性,这类化合物具有开发成为抗癌剂的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号