当前位置:
X-MOL 学术
›
Nat. Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Allosteric sensitization of proapoptotic BAX
Nature Chemical Biology ( IF 12.9 ) Pub Date : 2017-07-10 00:00:00 , DOI: 10.1038/nchembio.2433 Jonathan R Pritz , Franziska Wachter , Susan Lee , James Luccarelli , Thomas E Wales , Daniel T Cohen , Paul Coote , Gregory J Heffron , John R Engen , Walter Massefski , Loren D Walensky
Nature Chemical Biology ( IF 12.9 ) Pub Date : 2017-07-10 00:00:00 , DOI: 10.1038/nchembio.2433 Jonathan R Pritz , Franziska Wachter , Susan Lee , James Luccarelli , Thomas E Wales , Daniel T Cohen , Paul Coote , Gregory J Heffron , John R Engen , Walter Massefski , Loren D Walensky
|
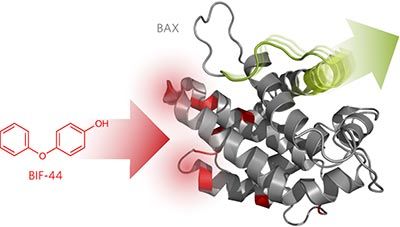
BCL-2-associated X protein (BAX) is a critical apoptotic regulator that can be transformed from a cytosolic monomer into a lethal mitochondrial oligomer, yet drug strategies to modulate it are underdeveloped due to longstanding difficulties in conducting screens on this aggregation-prone protein. Here, we overcame prior challenges and performed an NMR-based fragment screen of full-length human BAX. We identified a compound that sensitizes BAX activation by binding to a pocket formed by the junction of the α3–α4 and α5–α6 hairpins. Biochemical and structural analyses revealed that the molecule sensitizes BAX by allosterically mobilizing the α1–α2 loop and BAX BH3 helix, two motifs implicated in the activation and oligomerization of BAX, respectively. By engaging a region of core hydrophobic interactions that otherwise preserve the BAX inactive state, the identified compound reveals fundamental mechanisms for conformational regulation of BAX and provides a new opportunity to reduce the apoptotic threshold for potential therapeutic benefit.
中文翻译:

凋亡BAX的变构致敏
BCL-2-缔合的X蛋白(BAX)是一种关键的凋亡调节剂,可以从胞质单体转化为致死的线粒体寡聚体,但由于对这种易于凝集的蛋白进行筛查的长期困难,因此尚无法开发出调节其的药物策略。 。在这里,我们克服了先前的挑战,并对全长人类BAX进行了基于NMR的片段筛选。我们确定了通过结合到由所述的结形成的口袋敏感BAX活化的化合物α 3- α 4和α 5 α 6个发夹。生物化学和结构分析表明,该分子通过变构动员敏感BAX α 1- α2个环和BAX BH3螺旋,分别涉及BAX的激活和低聚的两个基序。通过接合核心疏水相互作用区域,否则该区域保留了BAX失活状态,所鉴定的化合物揭示了BAX构象调节的基本机制,并为降低潜在治疗益处的凋亡阈值提供了新的机会。
更新日期:2017-08-19
中文翻译:

凋亡BAX的变构致敏
BCL-2-缔合的X蛋白(BAX)是一种关键的凋亡调节剂,可以从胞质单体转化为致死的线粒体寡聚体,但由于对这种易于凝集的蛋白进行筛查的长期困难,因此尚无法开发出调节其的药物策略。 。在这里,我们克服了先前的挑战,并对全长人类BAX进行了基于NMR的片段筛选。我们确定了通过结合到由所述的结形成的口袋敏感BAX活化的化合物α 3- α 4和α 5 α 6个发夹。生物化学和结构分析表明,该分子通过变构动员敏感BAX α 1- α2个环和BAX BH3螺旋,分别涉及BAX的激活和低聚的两个基序。通过接合核心疏水相互作用区域,否则该区域保留了BAX失活状态,所鉴定的化合物揭示了BAX构象调节的基本机制,并为降低潜在治疗益处的凋亡阈值提供了新的机会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号