当前位置:
X-MOL 学术
›
Nat. Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structures of carboxylic acid reductase reveal domain dynamics underlying catalysis
Nature Chemical Biology ( IF 12.9 ) Pub Date : 2017-07-17 00:00:00 , DOI: 10.1038/nchembio.2434 Deepankar Gahloth , Mark S Dunstan , Daniela Quaglia , Evaldas Klumbys , Michael P Lockhart-Cairns , Andrew M Hill , Sasha R Derrington , Nigel S Scrutton , Nicholas J Turner , David Leys
Nature Chemical Biology ( IF 12.9 ) Pub Date : 2017-07-17 00:00:00 , DOI: 10.1038/nchembio.2434 Deepankar Gahloth , Mark S Dunstan , Daniela Quaglia , Evaldas Klumbys , Michael P Lockhart-Cairns , Andrew M Hill , Sasha R Derrington , Nigel S Scrutton , Nicholas J Turner , David Leys
|
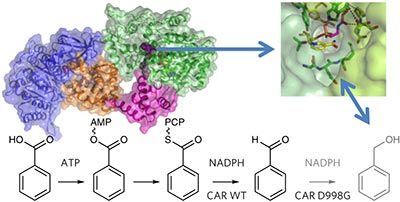
Carboxylic acid reductase (CAR) catalyzes the ATP- and NADPH-dependent reduction of carboxylic acids to the corresponding aldehydes. The enzyme is related to the nonribosomal peptide synthetases, consisting of an adenylation domain fused via a peptidyl carrier protein (PCP) to a reductase termination domain. Crystal structures of the CAR adenylation–PCP didomain demonstrate that large-scale domain motions occur between the adenylation and thiolation states. Crystal structures of the PCP–reductase didomain reveal that phosphopantetheine binding alters the orientation of a key Asp, resulting in a productive orientation of the bound nicotinamide. This ensures that further reduction of the aldehyde product does not occur. Combining crystallography with small-angle X-ray scattering (SAXS), we propose that molecular interactions between initiation and termination domains are limited to competing PCP docking sites. This theory is supported by the fact that (R)-pantetheine can support CAR activity for mixtures of the isolated domains. Our model suggests directions for further development of CAR as a biocatalyst.
中文翻译:

羧酸还原酶的结构揭示了催化作用下的结构域动力学
羧酸还原酶(CAR)催化ATP和NADPH依赖性的羧酸还原为相应的醛。该酶与非核糖体肽合成酶有关,该酶由通过肽基载体蛋白(PCP)与还原酶终止结构域融合的腺苷酸化结构域组成。CAR腺苷酸化-PCP双结构域的晶体结构表明,在腺苷酸化和硫醇化状态之间会发生大规模的畴运动。PCP-还原酶双结构域的晶体结构表明,磷酸泛酸的结合改变了关键Asp的方向,从而导致了结合的烟酰胺的生产性方向。这确保了不会进一步减少醛产物。将晶体学与小角度X射线散射(SAXS)结合起来,我们建议起始和终止域之间的分子相互作用仅限于竞争的PCP停靠位点。该理论得到以下事实的支持:R)-泛氨酸可以支持分离结构域混合物的CAR活性。我们的模型为CAR作为生物催化剂的进一步发展提供了方向。
更新日期:2017-08-19
中文翻译:

羧酸还原酶的结构揭示了催化作用下的结构域动力学
羧酸还原酶(CAR)催化ATP和NADPH依赖性的羧酸还原为相应的醛。该酶与非核糖体肽合成酶有关,该酶由通过肽基载体蛋白(PCP)与还原酶终止结构域融合的腺苷酸化结构域组成。CAR腺苷酸化-PCP双结构域的晶体结构表明,在腺苷酸化和硫醇化状态之间会发生大规模的畴运动。PCP-还原酶双结构域的晶体结构表明,磷酸泛酸的结合改变了关键Asp的方向,从而导致了结合的烟酰胺的生产性方向。这确保了不会进一步减少醛产物。将晶体学与小角度X射线散射(SAXS)结合起来,我们建议起始和终止域之间的分子相互作用仅限于竞争的PCP停靠位点。该理论得到以下事实的支持:R)-泛氨酸可以支持分离结构域混合物的CAR活性。我们的模型为CAR作为生物催化剂的进一步发展提供了方向。











































 京公网安备 11010802027423号
京公网安备 11010802027423号