当前位置:
X-MOL 学术
›
Nat. Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A water-soluble DsbB variant that catalyzes disulfide-bond formation in vivo
Nature Chemical Biology ( IF 12.9 ) Pub Date : 2017-06-19 00:00:00 , DOI: 10.1038/nchembio.2409 Dario Mizrachi , Michael-Paul Robinson , Guoping Ren , Na Ke , Mehmet Berkmen , Matthew P DeLisa
Nature Chemical Biology ( IF 12.9 ) Pub Date : 2017-06-19 00:00:00 , DOI: 10.1038/nchembio.2409 Dario Mizrachi , Michael-Paul Robinson , Guoping Ren , Na Ke , Mehmet Berkmen , Matthew P DeLisa
|
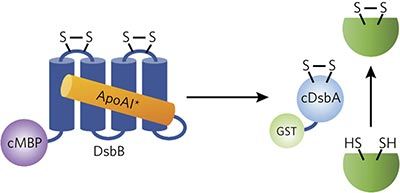
Escherichia coli DsbB is a transmembrane enzyme that catalyzes the reoxidation of the periplasmic oxidase DsbA by ubiquinone. Here, we sought to convert membrane-bound DsbB into a water-soluble biocatalyst by leveraging a previously described method for in vivo solubilization of integral membrane proteins (IMPs). When solubilized DsbB variants were coexpressed with an export-defective copy of DsbA in the cytoplasm of wild-type E. coli cells, artificial oxidation pathways were created that efficiently catalyzed de novo disulfide-bond formation in a range of substrate proteins, in a manner dependent on both DsbA and quinone. Hence, DsbB solubilization was achieved with preservation of both catalytic activity and substrate specificity. Moreover, given the generality of the solubilization technique, the results presented here should pave the way to unlocking the biocatalytic potential of other membrane-bound enzymes whose utility has been limited by poor stability of IMPs outside of their native lipid-bilayer context.
中文翻译:

水溶性DsbB变体,可在体内催化二硫键的形成
大肠杆菌DsbB是一种跨膜酶,可催化泛醌对周质氧化酶DsbA的再氧化。在这里,我们试图通过利用先前描述的体内整合膜蛋白(IMPs)的溶解方法,将结合膜的DsbB转化为水溶性生物催化剂。当在野生型大肠杆菌细胞质中将溶解的DsbB变体与DsbA的出口缺陷型拷贝共表达时,便产生了可以有效地从头催化的人工氧化途径取决于底物DsbA和醌的方式,在一定范围的底物蛋白质中形成二硫键。因此,在保持催化活性和底物特异性的情况下实现了DsbB增溶。此外,鉴于增溶技术的普遍性,此处介绍的结果应为释放其他膜结合酶的生物催化潜力铺平道路,这些酶的效用受到其天然脂质双分子层环境之外IMP稳定性差的限制。
更新日期:2017-08-19
中文翻译:

水溶性DsbB变体,可在体内催化二硫键的形成
大肠杆菌DsbB是一种跨膜酶,可催化泛醌对周质氧化酶DsbA的再氧化。在这里,我们试图通过利用先前描述的体内整合膜蛋白(IMPs)的溶解方法,将结合膜的DsbB转化为水溶性生物催化剂。当在野生型大肠杆菌细胞质中将溶解的DsbB变体与DsbA的出口缺陷型拷贝共表达时,便产生了可以有效地从头催化的人工氧化途径取决于底物DsbA和醌的方式,在一定范围的底物蛋白质中形成二硫键。因此,在保持催化活性和底物特异性的情况下实现了DsbB增溶。此外,鉴于增溶技术的普遍性,此处介绍的结果应为释放其他膜结合酶的生物催化潜力铺平道路,这些酶的效用受到其天然脂质双分子层环境之外IMP稳定性差的限制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号