Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
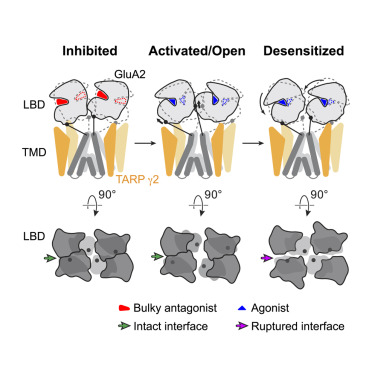
Activation and Desensitization Mechanism of AMPA Receptor-TARP Complex by Cryo-EM.
Cell ( IF 45.5 ) Pub Date : 2017-Sep-07 , DOI: 10.1016/j.cell.2017.07.045 Shanshuang Chen 1 , Yan Zhao 1 , Yuhang Wang 2 , Mrinal Shekhar 2 , Emad Tajkhorshid 2 , Eric Gouaux 3
Cell ( IF 45.5 ) Pub Date : 2017-Sep-07 , DOI: 10.1016/j.cell.2017.07.045 Shanshuang Chen 1 , Yan Zhao 1 , Yuhang Wang 2 , Mrinal Shekhar 2 , Emad Tajkhorshid 2 , Eric Gouaux 3
Affiliation
|
AMPA receptors mediate fast excitatory neurotransmission in the mammalian brain and transduce the binding of presynaptically released glutamate to the opening of a transmembrane cation channel. Within the postsynaptic density, however, AMPA receptors coassemble with transmembrane AMPA receptor regulatory proteins (TARPs), yielding a receptor complex with altered gating kinetics, pharmacology, and pore properties. Here, we elucidate structures of the GluA2-TARP γ2 complex in the presence of the partial agonist kainate or the full agonist quisqualate together with a positive allosteric modulator or with quisqualate alone. We show how TARPs sculpt the ligand-binding domain gating ring, enhancing kainate potency and diminishing the ensemble of desensitized states. TARPs encircle the receptor ion channel, stabilizing M2 helices and pore loops, illustrating how TARPs alter receptor pore properties. Structural and computational analysis suggests the full agonist and modulator complex harbors an ion-permeable channel gate, providing the first view of an activated AMPA receptor.
中文翻译:

Cryo-EM对AMPA受体-TARP复合物的激活和脱敏机制。
AMPA 受体介导哺乳动物大脑中的快速兴奋性神经传递,并将突触前释放的谷氨酸与跨膜阳离子通道的结合转导。然而,在突触后密度内,AMPA 受体与跨膜 AMPA 受体调节蛋白 (TARP) 共同组装,产生一种具有改变的门控动力学、药理学和孔特性的受体复合物。在这里,我们阐明了在部分激动剂红藻氨酸或完全激动剂 quisqualate 与正变构调节剂或仅与 quisqualate 一起存在的情况下 GluA2-TARP γ2 复合物的结构。我们展示了 TARPs 如何塑造配体结合域门控环,增强红藻氨酸效力并减少脱敏状态的集合。TARPs 包围受体离子通道,稳定 M2 螺旋和孔环,说明 TARPs 如何改变受体孔特性。结构和计算分析表明,完整的激动剂和调节剂复合物含有一个离子可渗透的通道门,提供了激活的 AMPA 受体的第一个视图。
更新日期:2017-08-17
中文翻译:

Cryo-EM对AMPA受体-TARP复合物的激活和脱敏机制。
AMPA 受体介导哺乳动物大脑中的快速兴奋性神经传递,并将突触前释放的谷氨酸与跨膜阳离子通道的结合转导。然而,在突触后密度内,AMPA 受体与跨膜 AMPA 受体调节蛋白 (TARP) 共同组装,产生一种具有改变的门控动力学、药理学和孔特性的受体复合物。在这里,我们阐明了在部分激动剂红藻氨酸或完全激动剂 quisqualate 与正变构调节剂或仅与 quisqualate 一起存在的情况下 GluA2-TARP γ2 复合物的结构。我们展示了 TARPs 如何塑造配体结合域门控环,增强红藻氨酸效力并减少脱敏状态的集合。TARPs 包围受体离子通道,稳定 M2 螺旋和孔环,说明 TARPs 如何改变受体孔特性。结构和计算分析表明,完整的激动剂和调节剂复合物含有一个离子可渗透的通道门,提供了激活的 AMPA 受体的第一个视图。











































 京公网安备 11010802027423号
京公网安备 11010802027423号