当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Blue Light Switchable Bacterial Adhesion as a Key Step toward the Design of Biofilms
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2017-08-17 00:00:00 , DOI: 10.1021/acssynbio.7b00197 Fei Chen 1 , Seraphine V. Wegner 1
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2017-08-17 00:00:00 , DOI: 10.1021/acssynbio.7b00197 Fei Chen 1 , Seraphine V. Wegner 1
Affiliation
|
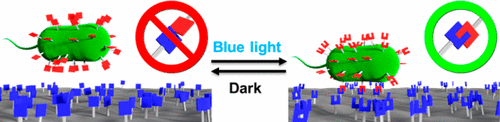
The control of where and when bacteria adhere to a substrate is a key step toward controlling the formation and organization in biofilms. This study shows how we engineer bacteria to adhere specifically to substrates with high spatial and temporal control under blue light, but not in the dark, by using photoswitchable interaction between nMag and pMag proteins. For this, we express pMag proteins on the surface of E. coli so that the bacteria can adhere to substrates with immobilized nMag protein under blue light. These adhesions are reversible in the dark and can be repeatedly turned on and off. Further, the number of bacteria that can adhere to the substrate as well as the attachment and detachment dynamics are adjustable by using different point mutants of pMag and altering light intensity. Overall, the blue light switchable bacteria adhesions offer reversible, tunable and bioorthogonal control with exceptional spatial and temporal resolution. This enables us to pattern bacteria on substrates with great flexibility.
中文翻译:

蓝光可转换细菌粘附力是生物膜设计的关键步骤
控制细菌附着在何时何处的基质是控制生物膜形成和组织的关键步骤。这项研究表明,我们如何利用nMag和pMag蛋白质之间的光开关相互作用,使细菌工程化,使其在蓝光下而不是在暗处具有高时空控制的底物上特异性粘附。为此,我们在大肠杆菌表面表达pMag蛋白因此细菌可以在蓝光下粘附到固定有nMag蛋白的底物上。这些附着力在黑暗中是可逆的,可以反复打开和关闭。此外,通过使用pMag的不同点突变体并改变光强度,可以调节可附着在基质上的细菌数量以及附着和分离动力学。总体而言,蓝光可转换细菌粘附力可逆,可调和生物正交控制,并具有出色的时空分辨率。这使我们能够以很大的灵活性在基底上对细菌进行图案化。
更新日期:2017-08-17
中文翻译:

蓝光可转换细菌粘附力是生物膜设计的关键步骤
控制细菌附着在何时何处的基质是控制生物膜形成和组织的关键步骤。这项研究表明,我们如何利用nMag和pMag蛋白质之间的光开关相互作用,使细菌工程化,使其在蓝光下而不是在暗处具有高时空控制的底物上特异性粘附。为此,我们在大肠杆菌表面表达pMag蛋白因此细菌可以在蓝光下粘附到固定有nMag蛋白的底物上。这些附着力在黑暗中是可逆的,可以反复打开和关闭。此外,通过使用pMag的不同点突变体并改变光强度,可以调节可附着在基质上的细菌数量以及附着和分离动力学。总体而言,蓝光可转换细菌粘附力可逆,可调和生物正交控制,并具有出色的时空分辨率。这使我们能够以很大的灵活性在基底上对细菌进行图案化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号