Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2017-08-15 , DOI: 10.1016/j.bmc.2017.08.022 Md. Saifuzzaman , Rick Morrison , Zhaohua Zheng , Stephanie Orive , Justin Hamilton , Philip E. Thompson , Jasim M.A. Al-rawi
|
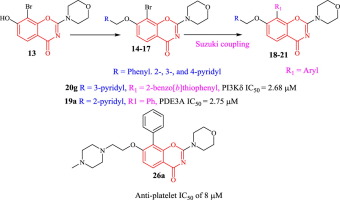
A series of 40 7-(O-substituted)-2-morpholino-8-aryl-4H-benzo[e][1,3]oxazin-4-one derivatives was synthesized. They were prepared via synthesis of a key precursor, 8-bromo-7-hydroxy-2-morpholino-4H-benzo[e][1,3]oxazin-4-one 13 which was amenable to ether synthesis at the 7-position and Suzuki coupling at the 8-position. The 2 protons of 7-OCH2 in compounds 18g, 18h, 18i, 18l and 18m prove to be magnetically non-equivalent, atropisomerism (axial chirality), as result of sterically hindered rotation of the bulky 8-aryl-substituent.
The products were evaluated for their activities against PI3K isoforms, DNA-PK and PDE3. The results showed that this substitution pattern has a deleterious effect on PI3K activities, which may arise from steric hindrance in the active site. PI3Kδ was somewhat more tolerant of this substitution particularly where 8-(4-methoxylphenyl) substituents were present (IC50s ∼ 2–3 μM). Good activities against PDE3 were also obtained for compounds, with particular members of the 7-(2-pyridinyl) methoxy series 19 showing good inhibition (IC50s ∼ 2–3 μM), comparable to previously described analogues. A piperazinyl derivative 26a effectively inhibited ADP-induced platelet aggregation with an IC50 of 8 μM.
中文翻译:

8-芳基-2-吗啉代-7-O-取代的苯并[ e ] [1,3]恶嗪-4-酮类化合物对DNA-PK,PI3K,PDE3A酶和血小板聚集的合成及生物学评价
合成了一系列40个7-(O-取代)-2-吗啉代-8-芳基-4H-苯并[ e ] [1,3]恶嗪-4-酮衍生物。它们是通过合成关键的前体8-溴7-羟基-2-吗啉代-4H-苯并[ e ] [1,3]恶嗪-4-酮13制备的,该化合物可在7位合成醚。和Suzuki联轴器在8位。7-OCH的2个质子2在化合物18克,18H,18I,18升和18米证明是不可磁当量,旋转对映异构(轴向手性),作为结果的位阻大体积的8 -芳基取代基的旋转。
评价产物对PI3K同工型,DNA-PK和PDE3的活性。结果表明,该取代模式对PI3K活性具有有害作用,这可能是由于活性位点的空间位阻引起的。PI3Kδ对这种取代的耐受性更高,特别是在存在8-(4-甲氧基苯基)取代基的情况下(IC 50 s〜2-3μM)。与上述类似物相比,化合物对PDE3的活性也很好,其中7-(2-吡啶基)甲氧基系列19的特定成员显示出良好的抑制作用(IC 50 s〜2-3μM)。哌嗪基衍生物26a有效抑制ADP诱导的血小板凝集,IC 50为8μM。











































 京公网安备 11010802027423号
京公网安备 11010802027423号