Journal of Controlled Release ( IF 10.5 ) Pub Date : 2017-08-15 , DOI: 10.1016/j.jconrel.2017.08.012 Akhilesh Kumar Shakya , Chang Hyun Lee , Harvinder Singh Gill
|
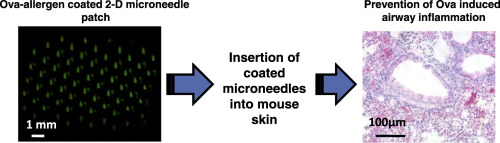
Allergy cases are increasing worldwide. Currently allergies are treated after their appearance in patients. However, now there is effort to make a preventive vaccine against allergies. The rationale is to target patient populations that are already sensitized to allergens but have yet to develop severe forms of the allergic disease, or who are susceptible to allergy development but have not yet developed them. Subcutaneous injections and the sublingual route have been used as the primary mode of preventive vaccine delivery. However, injections are painful, especially considering that they have to be given repeatedly to infants or young children. The sublingual route is hard to use since infants can't be trained to hold the vaccine under their tongue. In the present study, we demonstrate a microneedle (MN)-based cutaneous preventive allergy treatment against ovalbumin (Ova)-induced airway allergy in mice. Insertion of MNs coated with Ova as a model allergen and CpG oligonucleotide as an adjuvant (MNs-CIT) into the skin significantly induced Ova specific systemic immune response. This response was similar to that induced by hypodermic-needle-based delivery of Ova using the clinically-approved subcutaneous immunotherapy (SCIT) route. MNs-CIT regulated Th2 cytokines (IL-4, IL-5 & IL-13) and anti-inflammatory cytokines (IL-10) in the bronchoalveolar fluid, and IL-2 and IFN-γ cytokines in restimulated splenocyte cultures. Absence of mucus deposition inside the bronchiole wall and low collagen around the lung bronchioles after Ova-allergen challenge further confirmed the protective role of MNs-CIT. Overall, MNs-CIT represents a novel minimally invasive cutaneous immunotherapy to prevent the progression of Ova induced airway allergy in mice.
中文翻译:

涂有涂层的微针进行皮肤疫苗接种可预防气道过敏的发展
全球过敏病例在增加。目前,过敏症在患者出现后就得到了治疗。但是,现在正在努力制备预防过敏的疫苗。基本原理是针对已经对过敏原敏感但尚未发展为严重形式的过敏性疾病,或易患过敏症但尚未发展为过敏性疾病的患者人群。皮下注射和舌下途径已被用作预防性疫苗递送的主要方式。然而,注射是痛苦的,特别是考虑到必须反复给婴儿或幼儿注射。舌下途径很难使用,因为不能训练婴儿将疫苗接种在舌头下。在目前的研究中,我们证明了基于微针(MN)的针对卵白蛋白(Ova)诱导的小鼠气道过敏的皮肤预防过敏治疗。将包有Ova的MNs作为模型过敏原和CpG寡核苷酸作为佐剂(MNs-CIT)插入皮肤会明显诱导Ova特异性全身免疫反应。这种反应类似于使用临床上认可的皮下免疫疗法(SCIT)的基于皮下注射针的Ova诱导的反应。MNs-CIT调节支气管肺泡液中的Th2细胞因子(IL-4,IL-5和IL-13)和抗炎细胞因子(IL-10),以及再刺激的脾细胞培养物中的IL-2和IFN-γ细胞因子。Ova-变应原攻击后,细支气管壁内无粘液沉积,肺细支气管周围胶原含量低,进一步证实了MNs-CIT的保护作用。全面的,











































 京公网安备 11010802027423号
京公网安备 11010802027423号