当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Concentration-Driven Assembly and Sol–Gel Transition of π-Conjugated Oligopeptides
ACS Central Science ( IF 12.7 ) Pub Date : 2017-08-17 00:00:00 , DOI: 10.1021/acscentsci.7b00260 Yuecheng Zhou 1 , Bo Li 1 , Songsong Li 1 , Herdeline Ann M Ardoña 2 , William L Wilson 1, 3 , John D Tovar 2, 2 , Charles M Schroeder 1, 1
ACS Central Science ( IF 12.7 ) Pub Date : 2017-08-17 00:00:00 , DOI: 10.1021/acscentsci.7b00260 Yuecheng Zhou 1 , Bo Li 1 , Songsong Li 1 , Herdeline Ann M Ardoña 2 , William L Wilson 1, 3 , John D Tovar 2, 2 , Charles M Schroeder 1, 1
Affiliation
|
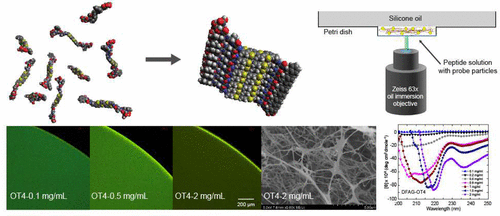
Advances in supramolecular assembly have enabled the design and synthesis of functional materials with well-defined structures across multiple length scales. Biopolymer-synthetic hybrid materials can assemble into supramolecular structures with a broad range of structural and functional diversity through precisely controlled noncovalent interactions between subunits. Despite recent progress, there is a need to understand the mechanisms underlying the assembly of biohybrid/synthetic molecular building blocks, which ultimately control the emergent properties of hierarchical assemblies. In this work, we study the concentration-driven self-assembly and gelation of π-conjugated synthetic oligopeptides containing different π-conjugated cores (quaterthiophene and perylene diimide) using a combination of particle tracking microrheology, confocal fluorescence microscopy, optical spectroscopy, and electron microscopy. Our results show that π-conjugated oligopeptides self-assemble into β-sheet-rich fiber-like structures at neutral pH, even in the absence of electrostatic screening of charged residues. A critical fiber formation concentration cfiber and a critical gel concentration cgel are determined for fiber-forming π-conjugated oligopeptides, and the linear viscoelastic moduli (storage modulus G′ and loss modulus G″) are determined across a wide range of peptide concentrations. These results suggest that the underlying chemical structure of the synthetic π-conjugated cores greatly influences the self-assembly process, such that oligopeptides appended to π-conjugated cores with greater torsional flexibility tend to form more robust fibers upon increasing peptide concentration compared to oligopeptides with sterically constrained cores. Overall, our work focuses on the molecular assembly of π-conjugated oligopeptides driven by concentration, which is controlled by a combination of enthalpic and entropic interactions between oligopeptide subunits.
中文翻译:

π-共轭寡肽的浓度驱动组装和溶胶-凝胶转变
超分子组装的进步使得能够设计和合成在多个长度尺度上具有明确结构的功能材料。生物聚合物合成杂化材料可以通过亚基之间精确控制的非共价相互作用组装成具有广泛结构和功能多样性的超分子结构。尽管最近取得了进展,但仍需要了解生物杂化/合成分子构件组装的潜在机制,这些机制最终控制分层组装的新兴特性。在这项工作中,我们结合粒子追踪微流变学、共焦荧光显微镜、光谱和电子技术,研究了含有不同 π 共轭核心(四噻吩和苝二酰亚胺)的 π 共轭合成寡肽的浓度驱动自组装和凝胶化。显微镜。我们的结果表明,即使在没有对带电残基进行静电屏蔽的情况下,π-共轭寡肽在中性 pH 值下也会自组装成富含 β-折叠的纤维状结构。确定了形成纤维的 π-共轭寡肽的临界纤维形成浓度c Fiber和临界凝胶浓度c gel ,并确定了各种肽浓度下的线性粘弹性模量(储能模量G ' 和损耗模量G '') 。 这些结果表明,合成π共轭核心的基本化学结构极大地影响自组装过程,使得附加到具有更大扭转灵活性的π共轭核心的寡肽在增加肽浓度时倾向于形成更坚固的纤维,与具有更大扭转柔韧性的寡肽相比,空间约束的核心。总体而言,我们的工作重点是由浓度驱动的 π 共轭寡肽的分子组装,浓度由寡肽亚基之间的焓和熵相互作用的组合控制。
更新日期:2017-08-17
中文翻译:

π-共轭寡肽的浓度驱动组装和溶胶-凝胶转变
超分子组装的进步使得能够设计和合成在多个长度尺度上具有明确结构的功能材料。生物聚合物合成杂化材料可以通过亚基之间精确控制的非共价相互作用组装成具有广泛结构和功能多样性的超分子结构。尽管最近取得了进展,但仍需要了解生物杂化/合成分子构件组装的潜在机制,这些机制最终控制分层组装的新兴特性。在这项工作中,我们结合粒子追踪微流变学、共焦荧光显微镜、光谱和电子技术,研究了含有不同 π 共轭核心(四噻吩和苝二酰亚胺)的 π 共轭合成寡肽的浓度驱动自组装和凝胶化。显微镜。我们的结果表明,即使在没有对带电残基进行静电屏蔽的情况下,π-共轭寡肽在中性 pH 值下也会自组装成富含 β-折叠的纤维状结构。确定了形成纤维的 π-共轭寡肽的临界纤维形成浓度c Fiber和临界凝胶浓度c gel ,并确定了各种肽浓度下的线性粘弹性模量(储能模量G ' 和损耗模量G '') 。 这些结果表明,合成π共轭核心的基本化学结构极大地影响自组装过程,使得附加到具有更大扭转灵活性的π共轭核心的寡肽在增加肽浓度时倾向于形成更坚固的纤维,与具有更大扭转柔韧性的寡肽相比,空间约束的核心。总体而言,我们的工作重点是由浓度驱动的 π 共轭寡肽的分子组装,浓度由寡肽亚基之间的焓和熵相互作用的组合控制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号