当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bioisosteric replacement of central 1,2,4-oxadiazole ring of high affinity CB2 ligands by regioisomeric 1,3,4-oxadiazole ring
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2017-07-19 00:00:00 , DOI: 10.1039/c7md00296c Dominik Heimann 1, 2, 3 , Corinna Lueg 1, 2, 3 , Henk de Vries 4, 5, 6, 7, 8 , Bastian Frehland 1, 2, 3 , Dirk Schepmann 1, 2, 3 , Laura H. Heitman 4, 5, 6, 7, 8 , Bernhard Wünsch 1, 2, 3, 9, 10
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2017-07-19 00:00:00 , DOI: 10.1039/c7md00296c Dominik Heimann 1, 2, 3 , Corinna Lueg 1, 2, 3 , Henk de Vries 4, 5, 6, 7, 8 , Bastian Frehland 1, 2, 3 , Dirk Schepmann 1, 2, 3 , Laura H. Heitman 4, 5, 6, 7, 8 , Bernhard Wünsch 1, 2, 3, 9, 10
Affiliation
|
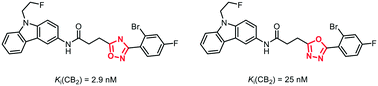
It has been reported that bioisosteric replacement of an 1,2,4-oxadiazole ring by an 1,3,4-oxadiazole ring leads to higher polarity, reduced metabolic degradation by human liver microsomes and reduced interaction with hERG channels. In a seven to eight step synthesis 1,3,4-oxadiazles 9a–c were synthesized as bioisosteric analogs of high-affinity but rather lipophilic CB2 ligands 1a–c containing an 1,2,4-oxadiazole ring. The 1,3,4-oxadiazole derivatives 9a and 9b show 10- and 50-fold reduced CB2 affinity compared to the 1,2,4-oxadiazole derivatives 1a and 1b, respectively. However, the 1,3,4-oxadiazole 9a has high CB2 affinity (Ki = 25 nM) and high selectivity over the CB1 receptor.
中文翻译:

高亲和力CB 2配体的中心1,2,4-恶二唑环被区域异构的1,3,4-恶二唑环取代
据报道,用1,3,4-恶二唑环生物等位取代1,2,4-恶二唑环可导致更高的极性,减少的人肝微粒体代谢降解以及减少与hERG通道的相互作用。在七到八步的合成过程中,合成了1,3,4-恶二唑9a-c作为具有高亲和力但亲脂性CB 2配体1a-c的生物立体异构体,其中CB 2配体1a-c含有1,2,4-恶二唑环。1,3,4-恶二唑衍生物9a和9b分别比1,2,4-恶二唑衍生物1a和1b降低了10倍和50倍的CB 2亲和力。但是1,3,4-恶二唑9a具有较高的CB 2亲和力(K i = 25 nM)和对CB 1受体的高选择性。
更新日期:2017-08-16
中文翻译:

高亲和力CB 2配体的中心1,2,4-恶二唑环被区域异构的1,3,4-恶二唑环取代
据报道,用1,3,4-恶二唑环生物等位取代1,2,4-恶二唑环可导致更高的极性,减少的人肝微粒体代谢降解以及减少与hERG通道的相互作用。在七到八步的合成过程中,合成了1,3,4-恶二唑9a-c作为具有高亲和力但亲脂性CB 2配体1a-c的生物立体异构体,其中CB 2配体1a-c含有1,2,4-恶二唑环。1,3,4-恶二唑衍生物9a和9b分别比1,2,4-恶二唑衍生物1a和1b降低了10倍和50倍的CB 2亲和力。但是1,3,4-恶二唑9a具有较高的CB 2亲和力(K i = 25 nM)和对CB 1受体的高选择性。










































 京公网安备 11010802027423号
京公网安备 11010802027423号