当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bitopic fluorescent antagonists of the A2A adenosine receptor based on pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine functionalized congeners
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2017-06-22 00:00:00 , DOI: 10.1039/c7md00247e Romain Duroux 1, 2, 3, 4, 5 , Antonella Ciancetta 1, 2, 3, 4, 5 , Philip Mannes 1, 2, 3, 4, 5 , Jinha Yu 1, 2, 3, 4, 5 , Shireesha Boyapati 1, 2, 3, 4, 5 , Elizabeth Gizewski 6, 7, 8, 9 , Said Yous 10, 11, 12, 13, 14 , Francisco Ciruela 15, 16, 17, 18, 19 , John A. Auchampach 6, 7, 8, 9 , Zhan-Guo Gao 1, 2, 3, 4, 5 , Kenneth A. Jacobson 1, 2, 3, 4, 5
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2017-06-22 00:00:00 , DOI: 10.1039/c7md00247e Romain Duroux 1, 2, 3, 4, 5 , Antonella Ciancetta 1, 2, 3, 4, 5 , Philip Mannes 1, 2, 3, 4, 5 , Jinha Yu 1, 2, 3, 4, 5 , Shireesha Boyapati 1, 2, 3, 4, 5 , Elizabeth Gizewski 6, 7, 8, 9 , Said Yous 10, 11, 12, 13, 14 , Francisco Ciruela 15, 16, 17, 18, 19 , John A. Auchampach 6, 7, 8, 9 , Zhan-Guo Gao 1, 2, 3, 4, 5 , Kenneth A. Jacobson 1, 2, 3, 4, 5
Affiliation
|
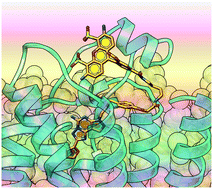
A pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine antagonist of the A2A adenosine receptor (AR) was functionalized as amine congeners, fluorescent conjugates and a sulfonate, and the A2AAR binding modes were predicted computationally. The optimal n-butyl spacer was incorporated into the following A2AAR-selective (Ki, nM) conjugates: BODIPY630/650 derivative 11 (MRS7396, 24.6) and AlexaFluor488 derivative 12 (MRS7416, 30.3). Flow cytometry of 12 in hA2AAR-expressing HEK-293 cells displayed saturable binding (low nonspecific) and inhibition by known A2AAR antagonists. Water-soluble sulfonate 13 was a highly potent (Ki = 6.2 nM) and selective A2AAR antagonist based on binding and functional assays. Docking and molecular dynamics simulations predicted the regions of interaction of the distal portions of these chain-extended ligands with the A2AAR. The BODIPY630/650 fluorophore of 11 was buried in a hydrophobic interhelical (TM1/TM7) region, while AlexaFluor488 of 12 associated with the hydrophilic extracellular loops. In conclusion, we have identified novel high affinity antagonist probes for A2AAR drug discovery and characterization.
中文翻译:

基于吡唑并[4,3- e ] [1,2,4]三唑并[1,5 - c ]嘧啶-5-胺官能化同源物的A 2A腺苷受体的双位荧光拮抗剂
A 2A腺苷受体(AR)的吡唑并[4,3- e ] [1,2,4]三唑并[1,5 - c ]嘧啶-5-胺拮抗剂被官能化为胺同类物,荧光共轭物和磺酸盐,并通过计算预测了A 2A AR结合模式。最佳正丁基间隔基被并入以下A 2A AR选择性(K i,nM)共轭物中:BODIPY630 / 650衍生物11(MRS7396,24.6)和AlexaFluor488衍生物12(MRS7416,30.3)。流式细胞仪检测hA 2A中的12表达AR的HEK-293细胞表现出饱和结合(低非特异性)和被已知的A 2A AR拮抗剂抑制。基于结合和功能测定,水溶性磺酸盐13是高效的(K i = 6.2 nM)和选择性的A 2A AR拮抗剂。对接和分子动力学模拟预测了这些扩链配体与A 2A AR相互作用的远端区域。11个的BODIPY630 / 650荧光团埋在疏水的螺旋间(TM1 / TM7)区域中,而12个的AlexaFluor488与亲水的细胞外环相关。总之,我们已经确定了针对A的新型高亲和力拮抗剂探针2A AR药物发现和表征。
更新日期:2017-08-16
中文翻译:

基于吡唑并[4,3- e ] [1,2,4]三唑并[1,5 - c ]嘧啶-5-胺官能化同源物的A 2A腺苷受体的双位荧光拮抗剂
A 2A腺苷受体(AR)的吡唑并[4,3- e ] [1,2,4]三唑并[1,5 - c ]嘧啶-5-胺拮抗剂被官能化为胺同类物,荧光共轭物和磺酸盐,并通过计算预测了A 2A AR结合模式。最佳正丁基间隔基被并入以下A 2A AR选择性(K i,nM)共轭物中:BODIPY630 / 650衍生物11(MRS7396,24.6)和AlexaFluor488衍生物12(MRS7416,30.3)。流式细胞仪检测hA 2A中的12表达AR的HEK-293细胞表现出饱和结合(低非特异性)和被已知的A 2A AR拮抗剂抑制。基于结合和功能测定,水溶性磺酸盐13是高效的(K i = 6.2 nM)和选择性的A 2A AR拮抗剂。对接和分子动力学模拟预测了这些扩链配体与A 2A AR相互作用的远端区域。11个的BODIPY630 / 650荧光团埋在疏水的螺旋间(TM1 / TM7)区域中,而12个的AlexaFluor488与亲水的细胞外环相关。总之,我们已经确定了针对A的新型高亲和力拮抗剂探针2A AR药物发现和表征。











































 京公网安备 11010802027423号
京公网安备 11010802027423号