当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Characterization of a highly efficient antibiotic-degrading metallo-β-lactamase obtained from an uncultured member of a permafrost community
Metallomics ( IF 2.9 ) Pub Date : 2017-07-18 00:00:00 , DOI: 10.1039/c7mt00195a Marcelo Monteiro Pedroso 1, 2, 3, 4 , Christopher Selleck 1, 2, 3, 4 , Charmaine Enculescu 1, 2, 3, 4 , Jeffrey R. Harmer 2, 3, 4, 5 , Nataša Mitić 6, 7, 8, 9 , Whitney R. Craig 10, 11, 12, 13 , Waleed Helweh 10, 13, 14, 15 , Philip Hugenholtz 1, 2, 3, 4, 16 , Gene W. Tyson 1, 2, 3, 4, 16 , David L. Tierney 10, 11, 12, 13 , James A. Larrabee 10, 13, 14, 15 , Gerhard Schenk 1, 2, 3, 4
Metallomics ( IF 2.9 ) Pub Date : 2017-07-18 00:00:00 , DOI: 10.1039/c7mt00195a Marcelo Monteiro Pedroso 1, 2, 3, 4 , Christopher Selleck 1, 2, 3, 4 , Charmaine Enculescu 1, 2, 3, 4 , Jeffrey R. Harmer 2, 3, 4, 5 , Nataša Mitić 6, 7, 8, 9 , Whitney R. Craig 10, 11, 12, 13 , Waleed Helweh 10, 13, 14, 15 , Philip Hugenholtz 1, 2, 3, 4, 16 , Gene W. Tyson 1, 2, 3, 4, 16 , David L. Tierney 10, 11, 12, 13 , James A. Larrabee 10, 13, 14, 15 , Gerhard Schenk 1, 2, 3, 4
Affiliation
|
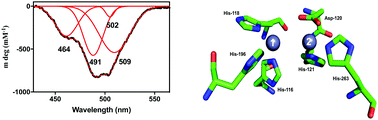
Antibiotic resistance is a major global health problem, one that threatens to derail the benefits garnered from arguably the greatest success of modern medicine, the discovery of antibiotics. Among the most potent agents contributing to antibiotic resistance are metallo-β-lactamases (MBLs). The discovery of MBL-like enzymes in microorganisms that are not in contact with the human population is of particular concern as these proteins already have the in-built capacity to inactivate antibiotics, even though they may not need MBL activity for their survival. Here, we demonstrate that a microbiome from a remote and frozen environment in Alaska harbours at least one highly efficient MBL, LRA-8. LRA-8 is homologous to the B3 subgroup of MBLs and has a substrate profile and catalytic properties similar to well-known members of this enzyme family, which are expressed by major human pathogens. LRA-8 is predominantly a penicillinase, but is also active towards carbapenems, but not cephalosporins. Spectroscopic studies indicate that LRA-8 has an active site structure similar to that of other MBLs (in particular B3 subgroup representative AIM-1), and a combination of steady-state and pre-steady-state kinetic data demonstrate that the enzyme is likely to employ a metal ion-bridging hydroxide to initiate catalysis. The rate-limiting step is the decay of a chromophoric, tetrahedral intermediate, as is observed in various other MBLs. Thus, studying the properties of such “pristine” MBL-like proteins may provide insight into the structural plasticity of this family of enzymes that may facilitate functional promiscuity, while important insight into the evolution of MBLs may also be gained.
中文翻译:

从永冻土群落未培养成员获得的高效抗生素降解金属β-内酰胺酶的表征
抗生素抗药性是全球性的主要健康问题,有可能使现代医学的最大成功(即发现抗生素)带来的益处脱轨。促成抗生素耐药性的最有效药物是金属β-内酰胺酶(MBL)。在不与人类接触的微生物中发现MBL样酶特别令人关注,因为这些蛋白已经具有内置的灭活抗生素的能力,即使它们可能不需要MBL活性即可生存。在这里,我们证明了一个来自阿拉斯加偏远和冰冻环境的微生物组至少具有一个高效的MBL LRA-8。LRA-8与MBL的B3亚基同源,并且具有类似于该酶家族众所周知的成员的底物特征和催化特性,由主要的人类病原体表达。LRA-8主要是青霉素酶,但对碳青霉烯类也有活性,但对头孢菌素没有活性。光谱研究表明,LRA-8具有类似于其他MBL(特别是B3亚组代表AIM-1)的活性位点结构,并且稳态和稳态前动力学数据的组合表明该酶很可能使用金属离子桥联氢氧化物引发催化作用。限速步骤是发色的四面体中间体的衰变,正如在其他各种MBL中所观察到的那样。因此,研究此类“原始” MBL样蛋白的特性可能提供对该酶家族的结构可塑性的洞察,这可能有助于功能混杂,同时也可能获得对MBLs进化的重要见解。
更新日期:2017-08-16
中文翻译:

从永冻土群落未培养成员获得的高效抗生素降解金属β-内酰胺酶的表征
抗生素抗药性是全球性的主要健康问题,有可能使现代医学的最大成功(即发现抗生素)带来的益处脱轨。促成抗生素耐药性的最有效药物是金属β-内酰胺酶(MBL)。在不与人类接触的微生物中发现MBL样酶特别令人关注,因为这些蛋白已经具有内置的灭活抗生素的能力,即使它们可能不需要MBL活性即可生存。在这里,我们证明了一个来自阿拉斯加偏远和冰冻环境的微生物组至少具有一个高效的MBL LRA-8。LRA-8与MBL的B3亚基同源,并且具有类似于该酶家族众所周知的成员的底物特征和催化特性,由主要的人类病原体表达。LRA-8主要是青霉素酶,但对碳青霉烯类也有活性,但对头孢菌素没有活性。光谱研究表明,LRA-8具有类似于其他MBL(特别是B3亚组代表AIM-1)的活性位点结构,并且稳态和稳态前动力学数据的组合表明该酶很可能使用金属离子桥联氢氧化物引发催化作用。限速步骤是发色的四面体中间体的衰变,正如在其他各种MBL中所观察到的那样。因此,研究此类“原始” MBL样蛋白的特性可能提供对该酶家族的结构可塑性的洞察,这可能有助于功能混杂,同时也可能获得对MBLs进化的重要见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号