当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper-finger protein of Sp1: the molecular basis of copper sensing
Metallomics ( IF 2.9 ) Pub Date : 2017-07-18 00:00:00 , DOI: 10.1039/c7mt00184c Siming Yuan 1, 2, 3, 4, 5 , Siming Chen 1, 2, 3, 4, 5 , Zhaoyong Xi 1, 2, 3, 4, 5 , Yangzhong Liu 1, 2, 3, 4, 5
Metallomics ( IF 2.9 ) Pub Date : 2017-07-18 00:00:00 , DOI: 10.1039/c7mt00184c Siming Yuan 1, 2, 3, 4, 5 , Siming Chen 1, 2, 3, 4, 5 , Zhaoyong Xi 1, 2, 3, 4, 5 , Yangzhong Liu 1, 2, 3, 4, 5
Affiliation
|
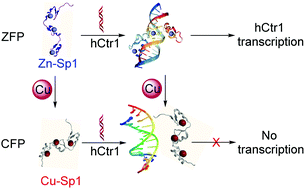
The cellular copper level is strictly regulated since excessive copper is harmful to cells. It has been proposed that the expression of copper transport protein hCtr1 is transcriptionally regulated by specificity protein 1 (Sp1) in response to the cellular copper level. However, it is not known how Sp1, a zinc-finger-protein (ZFP), can sense copper ions in cells. Here we found that Sp1 demonstrates high binding affinity to cuprous ions, even stronger than Cu-Atox1 binding. Cu(I) can displace Zn(II) in Sp1, resulting in a well-folded ‘Copper-Finger-Protein’ (CFP). Although only very little structural alteration occurs upon copper binding, CFP cannot recognize the promoter of hCtr1, therefore copper binding interrupts the transcription. This result indicates that, in addition to apo-to-holo alteration, metal substitution can also lead to transcriptional switch in metal sensing. This work provides insight into the copper sensing mechanism of Sp1 at the molecular level.
中文翻译:

Sp1的铜指蛋白:铜感测的分子基础
由于过量的铜对细胞有害,因此严格控制了细胞的铜水平。已经提出,响应于细胞铜水平,铜转运蛋白hCtr1的表达受特异性蛋白1(Sp1)的转录调控。但是,还不知道Sp1是一种锌指蛋白(ZFP)如何感应细胞中的铜离子。在这里,我们发现Sp1表现出对亚铜离子的高结合亲和力,甚至比Cu-Atox1结合更强。Cu(I)可以取代Sp1中的Zn(II),从而形成折叠良好的“铜指蛋白”(CFP)。虽然只有很少的结构改变发生在铜结合时,CFP无法识别hCtr1的启动子因此,铜结合中断了转录。该结果表明,除了脱辅基到全环的改变之外,金属取代还可以导致金属感测中的转录转换。这项工作提供了在分子水平上对Sp1铜感测机制的洞察力。
更新日期:2017-08-16
中文翻译:

Sp1的铜指蛋白:铜感测的分子基础
由于过量的铜对细胞有害,因此严格控制了细胞的铜水平。已经提出,响应于细胞铜水平,铜转运蛋白hCtr1的表达受特异性蛋白1(Sp1)的转录调控。但是,还不知道Sp1是一种锌指蛋白(ZFP)如何感应细胞中的铜离子。在这里,我们发现Sp1表现出对亚铜离子的高结合亲和力,甚至比Cu-Atox1结合更强。Cu(I)可以取代Sp1中的Zn(II),从而形成折叠良好的“铜指蛋白”(CFP)。虽然只有很少的结构改变发生在铜结合时,CFP无法识别hCtr1的启动子因此,铜结合中断了转录。该结果表明,除了脱辅基到全环的改变之外,金属取代还可以导致金属感测中的转录转换。这项工作提供了在分子水平上对Sp1铜感测机制的洞察力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号