当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Oxygen-dependent activation of Cu,Zn-superoxide dismutase-1
Metallomics ( IF 3.4 ) Pub Date : 2017-06-27 00:00:00 , DOI: 10.1039/c6mt00298f Morgan M. Fetherolf 1, 2, 3 , Stefanie D. Boyd 3, 4, 5, 6 , Duane D. Winkler 3, 4, 5, 6 , Dennis R. Winge 1, 2, 3
Metallomics ( IF 3.4 ) Pub Date : 2017-06-27 00:00:00 , DOI: 10.1039/c6mt00298f Morgan M. Fetherolf 1, 2, 3 , Stefanie D. Boyd 3, 4, 5, 6 , Duane D. Winkler 3, 4, 5, 6 , Dennis R. Winge 1, 2, 3
Affiliation
|
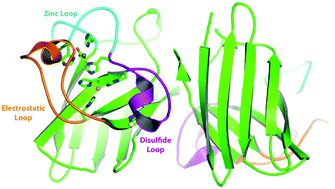
Copper zinc superoxide dismutase (Sod1) is a critical enzyme in limiting reactive oxygen species in both the cytosol and the mitochondrial intermembrane space. Sod1 dismutes superoxide anions to hydrogen peroxide and oxygen. The catalytic reaction is dependent on an active site copper ion and a disulfide bonded conformation. The activation of Sod1 is mediated by its chaperone Ccs1. The mechanism of Ccs1-mediated Sod1 activation involves both insertion of the catalytic copper ion and mediating disulfide bond formation. Since Sod1 is a highly abundant enzyme residing within the highly reducing cytoplasm, the question of disulfide bond formation is significant yet unresolved. The processes involved in Sod1 activation are reviewed with a focus on copper ion insertion and disulfide bond formation.
中文翻译:

Cu,Zn-超氧化物歧化酶-1的氧依赖性激活
铜锌超氧化物歧化酶(Sod1)是限制细胞质和线粒体膜间空间中活性氧种类的关键酶。Sod1将超氧阴离子歧化为过氧化氢和氧气。催化反应取决于活性位点铜离子和二硫键结合的构象。Sod1的激活是由其伴侣Ccs1介导的。Ccs1介导的Sod1活化的机制涉及催化铜离子的插入和介导二硫键的形成。由于Sod1是高度还原的细胞质内存在的高度丰富的酶,因此二硫键形成的问题很重要,但尚未解决。Sod1激活涉及的过程进行了审查,重点是铜离子插入和二硫键的形成。
更新日期:2017-08-16
中文翻译:

Cu,Zn-超氧化物歧化酶-1的氧依赖性激活
铜锌超氧化物歧化酶(Sod1)是限制细胞质和线粒体膜间空间中活性氧种类的关键酶。Sod1将超氧阴离子歧化为过氧化氢和氧气。催化反应取决于活性位点铜离子和二硫键结合的构象。Sod1的激活是由其伴侣Ccs1介导的。Ccs1介导的Sod1活化的机制涉及催化铜离子的插入和介导二硫键的形成。由于Sod1是高度还原的细胞质内存在的高度丰富的酶,因此二硫键形成的问题很重要,但尚未解决。Sod1激活涉及的过程进行了审查,重点是铜离子插入和二硫键的形成。



























 京公网安备 11010802027423号
京公网安备 11010802027423号