PLOS Medicine ( IF 10.5 ) Pub Date : 2017-08-15 , DOI: 10.1371/journal.pmed.1002377 Stefan A. Unger , Saikou Drammeh , Jahid Hasan , Kabiru Ceesay , Edrisa Sinjanka , Sainey Beyai , Bakary Sonko , Bai Lamin Dondeh , Anthony J. Fulford , Sophie E. Moore , Andrew M. Prentice
|
Background
Multiple micronutrients (MMN) are commonly prescribed in pediatric primary healthcare in sub-Saharan Africa to improve nutritional status and appetite without evidence for their effectiveness or international clinical guidelines. Community-wide MMN supplementation has shown limited and heterogeneous impact on growth and morbidity. Short-term ready-to-use therapeutic foods in acutely sick children in a hospital setting also had limited efficacy regarding subsequent growth. The effectiveness of MMN in improving morbidity or growth in sick children presenting for primary care has not been assessed.
Methods and findings
We undertook a double-blind randomised controlled trial of small-quantity lipid-based nutrient supplements (SQ-LNS) fortified with 23 micronutrients in children aged 6 months (mo) to 5 years (y) presenting with an illness at a rural primary healthcare centre in The Gambia. Primary outcomes were repeat clinic presentations and growth over 24 wk. Participants were randomly assigned to receive 1 of 3 interventions: (1) supplementation with micronutrient-fortified SQ-LNS for 12 wk (MMN-12), (2) supplementation with micronutrient-fortified SQ-LNS for 6 wk followed by unfortified SQ-LNS for 6 wk (MMN-6), or (3) supplementation with unfortified SQ-LNS for 12 wk (MMN-0) to be consumed in daily portions. Treatment masking used 16 letters per 6-wk block in the randomisation process. Blinded intention-to-treat analysis based on a prespecified statistical analysis plan included all participants eligible and correctly enrolled.
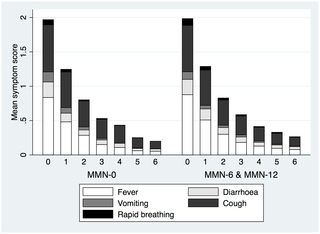
Between December 2009 and June 2011, 1,101 children (age 6–60 mo, mean 25.5 mo) were enrolled, and 1,085 were assessed (MMN-0 = 361, MMN-6 = 362, MMN-12 = 362). MMN supplementation was associated with a small increase in height-for-age z-scores 24 wk after recruitment (effect size for MMN groups combined: 0.084 SD/24 wk, 95% CI: 0.005, 0.168; p = 0.037; equivalent to 2–5 mm depending on age). No significant difference in frequency of morbidity measured by the number of visits to the clinic within 24 wk follow-up was detected with 0.09 presentations per wk for all groups (MMN-0 versus MMN-6: adjusted incidence rate ratio [IRR] 1.03, 95% CI: 0.92, 1.16; MMN-0 versus MMN-12: 1.05, 95% CI: 0.93, 1.18). In post hoc analysis, clinic visits significantly increased by 43% over the first 3 wk of fortified versus unfortified SQ-LNS (adjusted IRR 1.43; 95% CI: 1.07, 1.92; p = 0.016), with respiratory presentations increasing by 52% with fortified SQ-LNS (adjusted IRR 1.52; 95% CI: 1.01, 2.30; p = 0.046). The number of severe adverse events during supplementation were similar between groups (MMN-0 = 20 [1 death]; MMN-6 = 21 [1 death]; MMN-12 = 20 [0 death]). No participant withdrew due to adverse effects. Study limitations included the lack of supervision of daily supplementation.
Conclusion
Prescribing micronutrient-fortified SQ-LNS to ill children presenting for primary care in rural Gambia had a very small effect on linear growth and did not reduce morbidity compared to unfortified SQ-LNS. An early increase in repeat visits indicates a need for the establishment of evidence-based guidelines and caution with systematic prescribing of MMN. Future research should be directed at understanding the mechanisms behind the lack of effect of MMN supplementation on morbidity measures and limited effect on growth.
Trial registration
ISRCTN 73571031.
中文翻译:

强化和不强化脂类补充剂对发病率和营养状况的影响:冈比亚患病儿童的随机双盲安慰剂对照试验
背景
撒哈拉以南非洲地区的儿科初级保健中通常开有多种微量营养素(MMN),以改善营养状况和食欲,而没有证据表明其有效性或国际临床指南。整个社区的MMN补充剂对生长和发病率的影响均有限且不均一。在医院急症患儿中,短期即用的治疗性食品在随后的生长方面也具有有限的功效。未评估MMN在改善就诊初级病人的患病儿童的发病率或生长方面的有效性。
方法和发现
我们进行了一项小剂量的基于脂质的营养补充剂(SQ-LNS)的双盲随机对照试验,该营养补充剂在6个月(mo)至5岁(y)的农村初级医疗机构患病的儿童中添加了23种微量营养素。冈比亚的中心。主要结局是重复的临床表现和超过24周的生长。参与者被随机分配接受3种干预措施中的1种:(1)补充12周微营养素强化SQ-LNS(MMN-12),(2)补充6周微营养素强化SQ-LNS,然后进行不强化SQ- LNS为6周(MMN-6),或(3)补充每日12点的未经强化的SQ-LNS(MMN-0)。在随机化过程中,治疗掩蔽每6周使用16个字母。
在2009年12月至2011年6月之间,招募了1,101名儿童(6-60岁,平均25.5岁),评估了1,085名儿童(MMN-0 = 361,MMN-6 = 362,MMN-12 = 362)。补充MMN与募集后24周的年龄高度z分数小幅增加相关(MMN组的合并效应量:0.084 SD / 24 wk,95%CI:0.005,0.168; p= 0.037; 相当于2到5毫米,视年龄而定)。对于所有组,每星期每0.09例报告,通过随访24周内就诊的次数没有发现发病率的显着差异(MMN-0与MMN-6:调整后的发生率之比[IRR] 1.03, 95%CI:0.92、1.16; MMN-0与MMN-12:1.05、95%CI:0.93、1.18)。在事后分析中,在强化和未强化的SQ-LNS的前3周内,临床就诊显着增加了43%(调整后的IRR 1.43; 95%CI:1.07、1.92;p = 0.016),随着强化SQ-LNS(调整后的内部收益率1.52; 95%CI:1.01、2.30;p= 0.046)。补充期间严重不良事件的数量在各组之间相似(MMN-0 = 20 [1死亡]; MMN-6 = 21 [1死亡]; MMN-12 = 20 [0死亡])。没有参与者因为不良影响而退出。研究局限性包括缺乏日常补充的监督。
结论
与未经强化的SQ-LNS相比,对冈比亚农村地区就诊的患病儿童开加微量营养素强化的SQ-LNS对线性生长的影响很小,并且没有降低发病率。重复访问的尽早增加表明需要建立基于证据的指南,并要谨慎地对MMN进行系统处方。未来的研究应着眼于了解缺乏MMN补充对发病率措施的影响以及对生长的有限影响的机制。
试用注册
ISRCTN 73571031。











































 京公网安备 11010802027423号
京公网安备 11010802027423号