当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Clot-entrapped blood cells in synergy with human mesenchymal stem cells create a pro-angiogenic healing response
Biomaterials Science ( IF 5.8 ) Pub Date : 2017-08-15 00:00:00 , DOI: 10.1039/c7bm00276a Melanie A. Burkhardt 1, 2, 3, 4, 5 , Isabel Gerber 1, 2, 3, 4, 5 , Cameron Moshfegh 1, 2, 3, 4, 5 , Miriam S. Lucas 4, 5, 6, 7 , Jasmin Waser 8, 9, 10 , Maximilian Y. Emmert 5, 10, 11 , Simon P. Hoerstrup 5, 10, 11 , Falko Schlottig 8, 9, 10, 12, 13 , Viola Vogel 1, 2, 3, 4, 5
Biomaterials Science ( IF 5.8 ) Pub Date : 2017-08-15 00:00:00 , DOI: 10.1039/c7bm00276a Melanie A. Burkhardt 1, 2, 3, 4, 5 , Isabel Gerber 1, 2, 3, 4, 5 , Cameron Moshfegh 1, 2, 3, 4, 5 , Miriam S. Lucas 4, 5, 6, 7 , Jasmin Waser 8, 9, 10 , Maximilian Y. Emmert 5, 10, 11 , Simon P. Hoerstrup 5, 10, 11 , Falko Schlottig 8, 9, 10, 12, 13 , Viola Vogel 1, 2, 3, 4, 5
Affiliation
|
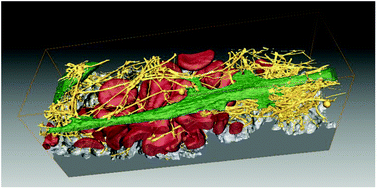
Blood clots stop bleeding and provide cell-instructive microenvironments. Still, in vitro models used to study implant performance typically neglect any possible interactions of recruited cells with surface-adhering blood clots. Here we study the interaction and synergies of bone marrow derived human mesenchymal stem cells (hMSCs) with surface-induced blood clots in an in vitro model by fluorescence microscopy, scanning and correlative light and electron microscopy, ELISA assays and zymography. The clinically used alkali-treated rough titanium (Ti) surfaces investigated here are known to enhance blood clotting compared to native Ti and to improve the healing response, but the underlying mechanisms remain elusive. Here we show that the presence of blood clots synergistically increased hMSC proliferation, extracellular matrix (ECM) remodelling and the release of matrix fragments and angiogenic VEGF, but did not increase the osteogenic differentiation of hMSCs. While many biomaterials are nowadays engineered to release pro-angiogenic factors, we show here that clot-entrapped blood cells on conventional materials in synergy with hMSCs are potent producers of pro-angiogenic factors. Our data might thus not only explain why alkali-treatment is beneficial for Ti implant integration, but they suggest that the physiological importance of blood clots to create pro-angiogenic environments on implants has been greatly underestimated. The importance of blood clots might have been missed because the pro-angiogenic functions get activated only upon stimulation by synergistic interactions with the invading cells.
中文翻译:

与人间充质干细胞协同作用的凝结血细胞产生促血管生成的愈合反应
血凝块可止血并提供具有细胞指导作用的微环境。尽管如此,用于研究植入物性能的体外模型通常会忽略募集的细胞与表面粘附的血凝块的任何可能的相互作用。在这里,我们研究了骨髓来源的人间充质干细胞(hMSCs)与表面诱导的血凝块在体外的相互作用和协同作用通过荧光显微镜,扫描和相关光电子显微镜,ELISA测定法和酶谱法建立模型。已知此处研究的临床使用的碱处理过的粗糙钛(Ti)表面与天然Ti相比可增强血液凝结并改善愈合反应,但潜在的机制仍然难以捉摸。在这里,我们显示血凝块的存在协同增加了hMSC的增殖,细胞外基质(ECM)重塑以及基质片段和血管生成性VEGF的释放,但并未增加hMSC的成骨分化。尽管当今许多生物材料经过工程设计以释放促血管生成因子,但我们在这里表明,与hMSC协同作用的常规材料上的血凝块包埋的血细胞是促血管生成因子的有效生产者。因此,我们的数据不仅可以解释为什么碱处理对钛植入物的整合有益,而且它们表明血凝块在植入物上形成促血管生成环境的生理重要性已被大大低估了。血凝块的重要性可能已经被忽略了,因为促血管生成功能只有在与侵入细胞的协同相互作用刺激后才被激活。
更新日期:2017-08-15
中文翻译:

与人间充质干细胞协同作用的凝结血细胞产生促血管生成的愈合反应
血凝块可止血并提供具有细胞指导作用的微环境。尽管如此,用于研究植入物性能的体外模型通常会忽略募集的细胞与表面粘附的血凝块的任何可能的相互作用。在这里,我们研究了骨髓来源的人间充质干细胞(hMSCs)与表面诱导的血凝块在体外的相互作用和协同作用通过荧光显微镜,扫描和相关光电子显微镜,ELISA测定法和酶谱法建立模型。已知此处研究的临床使用的碱处理过的粗糙钛(Ti)表面与天然Ti相比可增强血液凝结并改善愈合反应,但潜在的机制仍然难以捉摸。在这里,我们显示血凝块的存在协同增加了hMSC的增殖,细胞外基质(ECM)重塑以及基质片段和血管生成性VEGF的释放,但并未增加hMSC的成骨分化。尽管当今许多生物材料经过工程设计以释放促血管生成因子,但我们在这里表明,与hMSC协同作用的常规材料上的血凝块包埋的血细胞是促血管生成因子的有效生产者。因此,我们的数据不仅可以解释为什么碱处理对钛植入物的整合有益,而且它们表明血凝块在植入物上形成促血管生成环境的生理重要性已被大大低估了。血凝块的重要性可能已经被忽略了,因为促血管生成功能只有在与侵入细胞的协同相互作用刺激后才被激活。











































 京公网安备 11010802027423号
京公网安备 11010802027423号