当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Disordered N-Terminal Domain of Human Uracil DNA Glycosylase (hUNG2) Enhances DNA Translocation
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2017-08-15 00:00:00 , DOI: 10.1021/acschembio.7b00521 Gaddiel Rodriguez 1 , Alexandre Esadze 1 , Brian P. Weiser 1 , Joseph D. Schonhoft 1 , Philip A. Cole 1 , James T. Stivers 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2017-08-15 00:00:00 , DOI: 10.1021/acschembio.7b00521 Gaddiel Rodriguez 1 , Alexandre Esadze 1 , Brian P. Weiser 1 , Joseph D. Schonhoft 1 , Philip A. Cole 1 , James T. Stivers 1
Affiliation
|
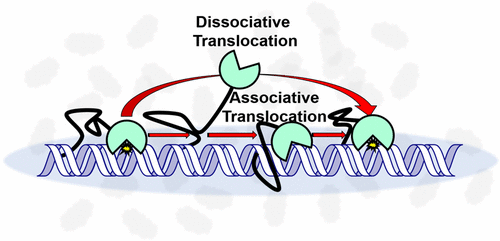
Nuclear human uracil–DNA glycosylase (hUNG2) initiates base excision repair (BER) of genomic uracils generated through misincorporation of dUMP or through deamination of cytosines. Like many human DNA glycosylases, hUNG2 contains an unstructured N-terminal domain that encodes a nuclear localization signal, protein binding motifs, and sites for post-translational modifications. Although the N-terminal domain has minimal effects on DNA binding and uracil excision kinetics, we report that this domain enhances the ability of hUNG2 to translocate on DNA chains as compared to the catalytic domain alone. The enhancement is most pronounced when physiological ion concentrations and macromolecular crowding agents are used. These data suggest that crowded conditions in the human cell nucleus promote the interaction of the N-terminus with duplex DNA during translocation. The increased contact time with the DNA chain likely contributes to the ability of hUNG2 to locate densely spaced uracils that arise during somatic hypermutation and during fluoropyrimidine chemotherapy.
中文翻译:

人尿嘧啶DNA糖基化酶(hUNG2)的无序N末端域增强DNA易位
人核尿嘧啶-DNA糖基化酶(hUNG2)启动通过dUMP掺入错误或通过胞嘧啶脱氨而产生的基因组尿嘧啶的碱基切除修复(BER)。像许多人类DNA糖基化酶一样,hUNG2包含一个非结构化的N末端结构域,该结构域编码核定位信号,蛋白结合基序和翻译后修饰位点。尽管N末端结构域对DNA结合和尿嘧啶切除动力学影响很小,但我们报道与单独的催化结构域相比,该结构域增强了hUNG2在DNA链上易位的能力。当使用生理离子浓度和大分子拥挤剂时,增强作用最为明显。这些数据表明,人细胞核中拥挤的状况在移位过程中会促进N末端与双链体DNA的相互作用。与DNA链的接触时间增加可能有助于hUNG2定位在体细胞高突变和氟嘧啶化疗期间出现的密集尿嘧啶的能力。
更新日期:2017-08-15
中文翻译:

人尿嘧啶DNA糖基化酶(hUNG2)的无序N末端域增强DNA易位
人核尿嘧啶-DNA糖基化酶(hUNG2)启动通过dUMP掺入错误或通过胞嘧啶脱氨而产生的基因组尿嘧啶的碱基切除修复(BER)。像许多人类DNA糖基化酶一样,hUNG2包含一个非结构化的N末端结构域,该结构域编码核定位信号,蛋白结合基序和翻译后修饰位点。尽管N末端结构域对DNA结合和尿嘧啶切除动力学影响很小,但我们报道与单独的催化结构域相比,该结构域增强了hUNG2在DNA链上易位的能力。当使用生理离子浓度和大分子拥挤剂时,增强作用最为明显。这些数据表明,人细胞核中拥挤的状况在移位过程中会促进N末端与双链体DNA的相互作用。与DNA链的接触时间增加可能有助于hUNG2定位在体细胞高突变和氟嘧啶化疗期间出现的密集尿嘧啶的能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号