当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thiol-Dependent Reduction of the Triester and Triamide Derivatives of Finland Trityl Radical Triggers O2-Dependent Superoxide Production
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2017-08-14 00:00:00 , DOI: 10.1021/acs.chemrestox.7b00086 Xiaoli Tan 1 , Li Chen 1 , Yuguang Song 1 , Antal Rockenbauer 2 , Frederick A. Villamena 3 , Jay L. Zweier 4 , Yangping Liu 1
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2017-08-14 00:00:00 , DOI: 10.1021/acs.chemrestox.7b00086 Xiaoli Tan 1 , Li Chen 1 , Yuguang Song 1 , Antal Rockenbauer 2 , Frederick A. Villamena 3 , Jay L. Zweier 4 , Yangping Liu 1
Affiliation
|
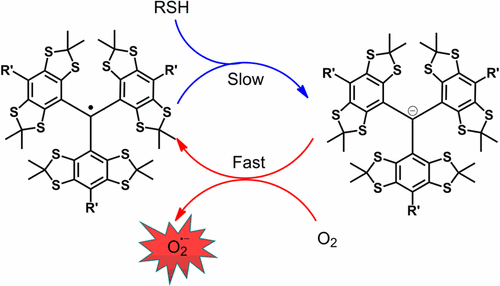
Tetrathiatriaylmethyl (trityl) radicals have found wide biomedical applications as magnetic resonance probes. Trityl radicals and their derivatives are generally stable toward biological reducing agents such as glutathione (GSH) and ascorbate. We demonstrate that the triester (ET-03) and triamide (AT-03) derivatives of the Finland trityl radical exhibit unique reduction by thiols such as GSH and cysteine (Cys) to generate the corresponding trityl carbanions as evidenced by the loss of EPR signal and appearance of characteristic UV–vis absorbance at 644 nm under anaerobic conditions. The trityl carbanions can be quickly converted back to the original trityl radicals by oxygen (O2) in air, thus rendering the reaction between the trityl derivative and biothiol undetectable under aerobic conditions. The reduction product of O2 by the trityl carbanions was shown to be superoxide radical (O2•–) by EPR spin-trapping. Kinetic studies showed that the reaction rate constants (k) depend on the types of both trityl radicals and thiols with the order of kET-03/Cys (0.336 M–1 s–1) > kET-03/GSH (0.070 M–1 s–1) > kAT-03/Cys (0.032 M–1 s–1) > kAT-03/GSH (0.027 M–1 s–1). The reactivity of trityl radicals with thiols is closely related to the para-substituents of trityl radicals as well as the pKa of the thiols and is further reflected by the rate of O2•– production and consumptions of O2 and thiols. This novel reaction represents a new metabolic process of trityl derivatives and should be considered in the design and application of new trityl radical probes.
中文翻译:

芬兰三苯甲基自由基的三酯和三酰胺衍生物的硫醇依赖性还原引发了O 2依赖性超氧化物的产生
四硫代三苯甲基(三苯甲基)自由基已被广泛用作磁共振探针的生物医学应用。三苯甲基自由基及其衍生物通常对生物还原剂(如谷胱甘肽(GSH)和抗坏血酸盐)稳定。我们证明芬兰三苯甲基自由基的三酯(ET-03)和三酰胺(AT-03)衍生物表现出独特的还原性,被巯基如GSH和半胱氨酸(Cys)生成相应的三苯甲基碳负离子,这由EPR信号的损失证明了厌氧条件下在644 nm处的特征UV-vis吸光度和外观。三苯甲基碳负离子可以通过氧气(O 2),因此在有氧条件下无法检测到三苯甲基衍生物与生物硫醇之间的反应。三苯甲基碳负离子对O 2的还原产物通过EPR自旋捕获显示为超氧自由基(O 2 •–)。动力学研究表明,反应速率常数(k)取决于三苯甲基自由基和硫醇的类型,顺序为k ET-03 / Cys(0.336 M –1 s –1)> k ET-03 / GSH(0.070 M –1 s –1)> k AT-03 / Cys(0.032 M –1 s –1)> kAT-03 / GSH(0.027 M –1 s –1)。三苯甲基自由基与硫醇的反应性与三苯甲基自由基的对位取代基以及硫醇的p K a密切相关,并进一步通过O 2 •的生成速率和O 2和硫醇的消耗来反映。这种新颖的反应代表了三苯甲基衍生物的新的代谢过程,应在新的三苯甲基自由基探针的设计和应用中加以考虑。
更新日期:2017-08-15
中文翻译:

芬兰三苯甲基自由基的三酯和三酰胺衍生物的硫醇依赖性还原引发了O 2依赖性超氧化物的产生
四硫代三苯甲基(三苯甲基)自由基已被广泛用作磁共振探针的生物医学应用。三苯甲基自由基及其衍生物通常对生物还原剂(如谷胱甘肽(GSH)和抗坏血酸盐)稳定。我们证明芬兰三苯甲基自由基的三酯(ET-03)和三酰胺(AT-03)衍生物表现出独特的还原性,被巯基如GSH和半胱氨酸(Cys)生成相应的三苯甲基碳负离子,这由EPR信号的损失证明了厌氧条件下在644 nm处的特征UV-vis吸光度和外观。三苯甲基碳负离子可以通过氧气(O 2),因此在有氧条件下无法检测到三苯甲基衍生物与生物硫醇之间的反应。三苯甲基碳负离子对O 2的还原产物通过EPR自旋捕获显示为超氧自由基(O 2 •–)。动力学研究表明,反应速率常数(k)取决于三苯甲基自由基和硫醇的类型,顺序为k ET-03 / Cys(0.336 M –1 s –1)> k ET-03 / GSH(0.070 M –1 s –1)> k AT-03 / Cys(0.032 M –1 s –1)> kAT-03 / GSH(0.027 M –1 s –1)。三苯甲基自由基与硫醇的反应性与三苯甲基自由基的对位取代基以及硫醇的p K a密切相关,并进一步通过O 2 •的生成速率和O 2和硫醇的消耗来反映。这种新颖的反应代表了三苯甲基衍生物的新的代谢过程,应在新的三苯甲基自由基探针的设计和应用中加以考虑。











































 京公网安备 11010802027423号
京公网安备 11010802027423号