PLOS Biology ( IF 7.8 ) Pub Date : 2017-08-14 , DOI: 10.1371/journal.pbio.2002354 Bing Xu , Ying Fu , Yan Liu , Sosse Agvanian , Robert C. Wirka , Rachel Baum , Kang Zhou , Robin M. Shaw , TingTing Hong
|
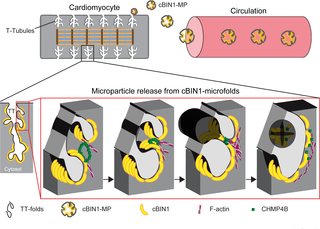
Microparticles (MPs) are cell–cell communication vesicles derived from the cell surface plasma membrane, although they are not known to originate from cardiac ventricular muscle. In ventricular cardiomyocytes, the membrane deformation protein cardiac bridging integrator 1 (cBIN1 or BIN1+13+17) creates transverse-tubule (t-tubule) membrane microfolds, which facilitate ion channel trafficking and modulate local ionic concentrations. The microfold- generated microdomains continuously reorganize, adapting in response to stress to modulate the calcium signaling apparatus. We explored the possibility that cBIN1-microfolds are externally released from cardiomyocytes. Using electron microscopy imaging with immunogold labeling, we found in mouse plasma that cBIN1 exists in membrane vesicles about 200 nm in size, which is consistent with the size of MPs. In mice with cardiac-specific heterozygous Bin1 deletion, flow cytometry identified 47% less cBIN1-MPs in plasma, supporting cardiac origin. Cardiac release was also evidenced by the detection of cBIN1-MPs in medium bathing a pure population of isolated adult mouse cardiomyocytes. In human plasma, osmotic shock increased cBIN1 detection by enzyme-linked immunosorbent assay (ELISA), and cBIN1 level decreased in humans with heart failure, a condition with reduced cardiac muscle cBIN1, both of which support cBIN1 release in MPs from human hearts. Exploring putative mechanisms of MP release, we found that the membrane fission complex endosomal sorting complexes required for transport (ESCRT)-III subunit charged multivesicular body protein 4B (CHMP4B) colocalizes and coimmunoprecipitates with cBIN1, an interaction enhanced by actin stabilization. In HeLa cells with cBIN1 overexpression, knockdown of CHMP4B reduced the release of cBIN1-MPs. Using truncation mutants, we identified that the N-terminal BAR (N-BAR) domain in cBIN1 is required for CHMP4B binding and MP release. This study links the BAR protein superfamily to the ESCRT pathway for MP biogenesis in mammalian cardiac ventricular cells, identifying elements of a pathway by which cytoplasmic cBIN1 is released into blood.
中文翻译:

ESCRT-III途径促进心肌细胞释放含cBIN1的微粒
微粒(MPs)是源自细胞表面质膜的细胞间通讯囊泡,尽管尚不知道它们源自心肌。在心室心肌细胞中,膜变形蛋白心脏桥整合子1(cBIN1或BIN1 + 13 + 17)产生横管(t管)膜微折叠,从而促进离子通道运输并调节局部离子浓度。微折叠产生的微区连续重组,响应压力而调节钙信号传导装置。我们探讨了cBIN1-microfolds从心肌细胞外部释放的可能性。使用带有免疫金标记的电子显微镜成像,我们在小鼠血浆中发现cBIN1存在于大小约为200 nm的膜囊泡中,这与MP的大小一致。仓1删除,流式细胞仪确定血浆中cBIN1-MPs减少47%,支持心脏起源。通过在培养分离出的成年小鼠心肌细胞的纯群体的培养基中检测到cBIN1-MPs,也可以证明心脏释放。在人血浆中,渗透性休克通过酶联免疫吸附测定(ELISA)增加了cBIN1的检测,而在患有心力衰竭的人中,cBIN1的水平下降,这是一种心肌cBIN1减少的疾病,两者都支持cBIN1在人心中从MP中释放。探索MP释放的推定机制,我们发现转运(ESCRT)-III亚基带电荷的多囊泡体蛋白4B(CHMP4B)所需的膜裂变复合物内体分选复合物与cBIN1共定位和共免疫沉淀,肌动蛋白稳定化增强了相互作用。在cBIN1过表达的HeLa细胞中,敲低CHMP4B减少cBIN1-MPs的释放。使用截断突变体,我们确定cBIN1中的N末端BAR(N-BAR)域是CHMP4B结合和MP释放所必需的。这项研究将BAR蛋白超家族与哺乳动物心脏心室细胞中MP生物发生的ESCRT途径相关联,从而确定了将胞质cBIN1释放到血液中的途径的元素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号