当前位置:
X-MOL 学术
›
Cancer Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Macrophage Polarization Contributes to Glioblastoma Eradication by Combination Immunovirotherapy and Immune Checkpoint Blockade.
Cancer Cell ( IF 48.8 ) Pub Date : 2017-08-14 , DOI: 10.1016/j.ccell.2017.07.006 Dipongkor Saha , Robert L. Martuza , Samuel D. Rabkin
Cancer Cell ( IF 48.8 ) Pub Date : 2017-08-14 , DOI: 10.1016/j.ccell.2017.07.006 Dipongkor Saha , Robert L. Martuza , Samuel D. Rabkin
|
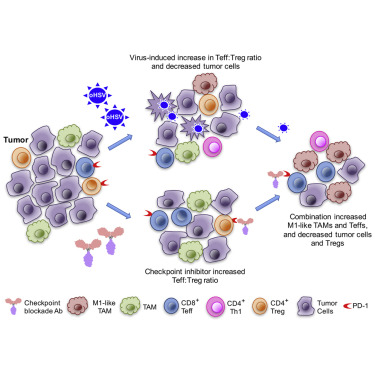
Glioblastoma is an immunosuppressive, fatal brain cancer that contains glioblastoma stem-like cells (GSCs). Oncolytic herpes simplex virus (oHSV) selectively replicates in cancer cells while inducing anti-tumor immunity. oHSV G47Δ expressing murine IL-12 (G47Δ-mIL12), antibodies to immune checkpoints (CTLA-4, PD-1, PD-L1), or dual combinations modestly extended survival of a mouse glioma model. However, the triple combination of anti-CTLA-4, anti-PD-1, and G47Δ-mIL12 cured most mice in two glioma models. This treatment was associated with macrophage influx and M1-like polarization, along with increased T effector to T regulatory cell ratios. Immune cell depletion studies demonstrated that CD4+ and CD8+ T cells as well as macrophages are required for synergistic curative activity. This combination should be translatable to the clinic and other immunosuppressive cancers.
中文翻译:

巨噬细胞极化通过免疫免疫疗法和免疫检查站封锁相结合,有助于根除胶质母细胞瘤。
胶质母细胞瘤是一种免疫抑制性致命性脑癌,其中包含胶质母细胞瘤干样细胞(GSC)。溶瘤性单纯疱疹病毒(oHSV)在癌细胞中选择性复制,同时诱导抗肿瘤免疫力。表达oHSVG47Δ的小鼠IL-12(G47Δ-mIL12),免疫检查点抗体(CTLA-4,PD-1,PD-L1)或双重组合适度延长了小鼠神经胶质瘤模型的生存期。但是,在两个神经胶质瘤模型中,抗CTLA-4,抗PD-1和G47Δ-mIL12的三联组合治愈了大多数小鼠。这种治疗与巨噬细胞的涌入和M1样极化有关,并伴随着T效应子与T调节细胞的比率增加。免疫细胞耗竭研究表明CD4 +和CD8 +T细胞和巨噬细胞是协同治疗的必要条件。这种组合应可转化为临床和其他免疫抑制性癌症。
更新日期:2017-08-14
中文翻译:

巨噬细胞极化通过免疫免疫疗法和免疫检查站封锁相结合,有助于根除胶质母细胞瘤。
胶质母细胞瘤是一种免疫抑制性致命性脑癌,其中包含胶质母细胞瘤干样细胞(GSC)。溶瘤性单纯疱疹病毒(oHSV)在癌细胞中选择性复制,同时诱导抗肿瘤免疫力。表达oHSVG47Δ的小鼠IL-12(G47Δ-mIL12),免疫检查点抗体(CTLA-4,PD-1,PD-L1)或双重组合适度延长了小鼠神经胶质瘤模型的生存期。但是,在两个神经胶质瘤模型中,抗CTLA-4,抗PD-1和G47Δ-mIL12的三联组合治愈了大多数小鼠。这种治疗与巨噬细胞的涌入和M1样极化有关,并伴随着T效应子与T调节细胞的比率增加。免疫细胞耗竭研究表明CD4 +和CD8 +T细胞和巨噬细胞是协同治疗的必要条件。这种组合应可转化为临床和其他免疫抑制性癌症。











































 京公网安备 11010802027423号
京公网安备 11010802027423号